Abstract
We investigated serum (IL-10 and IL-12p70) and cellular cytokine levels (IL-10, IL-12p40, IL-12p70, IFN-γ) in stimulated PBMC over 24 weeks in 15 Relapsing Remitting Multiple Sclerosis (MS) patients randomized to receive once-weekly (qw) IFN-β-1a 30 ug intramuscularly (IM) (n=8) or three-times-weekly (tiw) IFN-β-1a 44 ug subcutaneously (SC) (n=7). Overall, IFN-β treatment increased cellular IL-10 (p<0.01) levels and the ratios of cellular IL-10/IL-12p40 (p<0.01) and IL-10/IL-12p70 (p<0.02) while cellular IFN-γ levels were reduced (P<0.01). Serum IL-10 levels were decreased in non-responders to therapy based on MRI-defined criteria (p<0.01) but did not change in responders over the course of treatment. In addition, non-responders demonstrated a decrease in serum IL-10/IL-12p70 ratio (p=0.031) and a decrease in cellular IL-12p70 (p<0.02). A decrease in cellular IFN-γ was observed in responders (p=0.013). This is the first study that compares cytokine changes between the two IFN-β regimes and demonstrates that serum IL-10 levels decrease in those patients who continue to have active MRI lesions while on interferon-beta therapy.
Keywords: multiple sclerosis, interferon-beta, interleukin-10, interleukin-12, interferon-gamma, biomarker
1. Introduction
Multiple cytokines and immune cell subsets have been implicated in disease activity in MS. Current research suggests that no one cytokine or cell subset is wholly responsible for disease induction, relapse severity or remission, but instead that a complex interplay of factors controls disease activity. Heterodimeric IL-12 is mainly secreted by monocytes and dendritic cells as two proteins, IL-12p40 and IL-12p35 which combine to form biologically active IL-12p70. An alternate heterodimer, IL-23p19, binds to IL-12p40 to produce IL-23, which has been implicated in disease activity in murine models of MS. IL-12 acts as an inflammatory cytokine inducer through activation and maturation of Th1 cells secreting IFN-γ. IL-12p40 levels are increased in MS peripheral blood mononuclear cells (PBMC) and acute CNS plaques, while IL-12p35 levels are decreased (Windhagen et al.,1995, Van Boxel-Dezaire et al., 1999). IL-12 levels correlate with clinical measures of disease activity (EDSS) and presence of MRI lesions (Makhlouf et al., 2001). IL-10 is produced by dendritic cells, macrophages, B cells and T cells (especially of the Th2 type). IL-10 acts to decrease inflammatory cytokine production by macrophages (Imitola et al., 2005). IL-10 secretion by PBMC from MS patients is decreased prior to relapse and increased during remission (Rieckmann et al., 1994, Pelfrey et al., 2000, Waubant et al., 2001). Endogenous IL-10 levels may also influence severity of disease (Luomala et al., 2003, Van Boxel-Dezaire et al., 1999). IFN-γ is a cytokine product of NK and T cells (primarily of the Th1 type) that activates mononuclear cells and promotes Th1 cell development, especially in the presence of IL-12 (Imitola et al., 2005). IFN-γ treatment precipitated relapses in MS patients (Panitch et al., 1987), and levels of IFN-γ are increased prior to relapse (Beck et al., 1988, Becher et al., 1999). However, IFN-γ also has anti-inflammatory effects, primarily by opposing T cell proliferation and possibly inducing T cell apoptosis (Badovinac et al., 2000).
IFN-β has been shown to decrease clinical and MRI measures of disease activity in MS (Interferon Beta Study Group 1993, Paty et al., 1993, PRISMS 1998, Simon et al., 1999). IFN-β acts to increase IL-10 levels and reduce IL-12 levels in MS patients, thus improving the ratio of IL-10 to IL-12 (Correale et al., 1995, Rudick et al., 1996, Wang et al., 2000, Byrnes et al., 2002, Berghella et al., 2005, Ersoy et al., 2005). The mechanism of IL-12 suppression has been shown to depend on IL-10 induction (Wang et al., 2000). Increased CSF IL-10 levels during IFN-β treatment correlate with clinical response to therapy (Rudick et al., 1998). IFN-β also reduces IFN-γ production by PBMC and the number of IFN-γ secreting cells (Becher et al., 1999, Furlan et al., 2000, Berghella et al., 2005). Lower pretreatment IFN-γ and IL-12p35 mRNA levels have been reported to predict response to different IFN-β therapies (Petereit et al., 2002, Van Boxel-Dezaire et al., 2000). A genetic haplotype that influences IL-10 expression has also been reported to predict response to IFN-β (Wergeland et al., 2005). Various formulations of IFN-β are available and some studies suggest that higher doses may have improved clinical efficacy, but few studies to date have directly compared the influence of differing preparations on cytokine levels (Panitch et al., 2002, Durelli et al., 2002, Sega et al., 2004). We therefore conducted a prospective randomized study of serum and cellular cytokine levels comparing once-weekly (qw) to three times weekly (tiw) IFN-β-1a therapy and correlated the cytokine changes with MRI response to therapy.
2. Material and Methods
Patients
This study was conducted on a cohort of MS patients at the University of Maryland who participated in the multi-center EVIDENCE trial of IFN-β-1a treatment regimens in MS (Panitch et al., 2002). The protocol was reviewed and approved by the IRB and all patients gave written informed consent. Eligible patients were diagnosed with definite relapsing-remitting MS (Poser et al., 1983) and EDSS scores of 0-5.5 and had experienced at least two clinical relapses in the two years prior to enrollment. Details of study design have previously been reported (Panitch et al., 2002). Fifteen patients were randomized to receive either 30 μg once per week (qw) IM (Avonex®, Biogen Idec, Cambridge, MA; n=8) or 44 μg three times per week (tiw) SC (Rebif®, Serono International, Geneva, Switzerland) of IFN-β-1a. Patients were assigned to separate blinded evaluating physicians and un-blinded treating physicians. All MRI scans were interpreted by blinded evaluators (University of British Columbia MRI reading center).
Patients were screened four weeks prior to initiation of therapy with a complete history and physical exam, baseline serum samples, and proton-density/T2-weighted and pre-and post-gadolinium (Gd) T1-weighted MRI imaging. Neurological exams, imaging and serum samples were again obtained at baseline on day one of therapy and at scheduled 4 week intervals up to twenty-four weeks. Screening and baseline data were used as matched controls for each individual patient in this non-placebo controlled study. Methylprednisolone 1 g daily IV for three days was permitted at the discretion of the evaluating physician for relapses with objective findings.
MRI studies
MRI scans were performed and evaluated according to standardized criteria under the direction of the University of British Columbia MS/MRI Research Group. Scans were evaluated for T1, T2 and Combined Unique (CU) lesions (defined as an active lesion on T1 post-Gd or T2 sequences, or both, avoiding double counting). There was a clear dichotomy in outcome based on CU lesions with one group of patients having persistent activity with multiple CU lesions on several scans and another group with none or only one CU lesion on all post-treatment scans. “Non-responders” were defined as those patients having three or more CU lesions on any post-treatment scan.
Blood samples
Heparinized blood (30 cc) and serum samples were obtained from the patients pre-treatment and during treatment at each scheduled clinic visit. Serum samples were stored at -80°C. Serum concentrations of IL-10 and total IL-12p70 were determined in triplicate using commercially available ELISA kits with controls. Peripheral blood mononuclear cells (PBMC) were obtained by separation on Ficoll-Hypaque gradients. Two million PBMC per ml of complete RPMI media were cultured for 24 hr in 24 well plates in the presence of SAC (2ug/ml) plus IFN-γ (100 IU/ml) to induce IL-12 or left unstimulated. Cell free supernatants were then collected and evaluated for cytokine levels by ELISA. Staphylococcus Aureus C was purchased from Sigma (St. Louis, MO). Human ELISA Development systems (DuoSets) for IL-10, IL-12p40, IL-12p70, and IFN-γ were purchased from RγD Systems, Inc. The sensitivities of the ELISAs were approximately 5 pg/ml for IL-10, 2 pg/ml for p40, 2 pg/ml for p70, and 15 pg/ml for IFN-γ.
Statistical analysis
Statistical analysis was done using Prism version 3.0. Differences within groups (between patients’ samples at various time points) were evaluated using repeated measures ANOVA. Differences between treatment groups (IFN-β tiw versus qw) and clinical response groups (responders versus non-responders) were evaluated using unpaired t-tests with Welch’s correction for normally distributed data and Mann-Whitney tests for non-parametric measures. Normality of data was determined using Kolmogorov-Smirnow tests. Because of the small sample size, normally distributed data was subjected to post-hoc Mann-Whitney tests to verify significant findings. Two-tailed p-values less than 0.05 were considered significant.
3. Results
Response to IFN-β treatment
In the group of responders (n=10, 4 receiving 30 mcg qw, 6 receiving 44 mcg tiw), there were a total of two clinical exacerbations (one each in two patients in the qw group), one of which required IV methylprednisolone. In the group of non-responders (n=5, 4 receiving 30 mcg qw, 1 receiving 44 mcg tiw), there were a total of five clinical exacerbations (one each in two patients in the qw group and one patient in the tiw group, and two separate exacerbations in one patient in the qw group), requiring four separate courses of IV methylprednisolone (one each in three patients in the qw group and one patient in the tiw group). Prior to IFN-β therapy non-responders had a higher mean number of CU lesions (5.20 ± 3.28 (SE), n=5) than responders (0.90 + 0.46 (SE), n=10), but the difference was not significant. During the course of therapy, the mean number of CU lesions was 0.22 ± 0.06 in the group of responders and 4.47 ± 0.85 in the group of non-responders (p=0.008) (see Table 1). Analysis using the total number of CU lesions during the course of therapy yielded similar results.
Table 1.
Combined Unique (CU) MRI Lesions for Responders and Non-Responders to Interferon Therapy at Baseline (-1) through 24 weeks
| Patient | IFN-β | -1 | 4 | 8 | 12 | 16 | 20 | 24 | mean CU lesions on IFN | total CU lesions on IFN |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | qw | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.33 | 2 |
| 12 | qw | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0.17 | 1 |
| 17 | qw | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0.5 | 3 |
| 19 | qw | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0.5 | 3 |
| 1 | tiw | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.17 | 1 |
| 4 | tiw | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | tiw | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0.17 | 1 |
| 8 | tiw | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | tiw | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | tiw | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.33 | 2 |
| 5 | qw | 4 | 1 | 7 | 4 | 2 | 6 | 20 | 6.67 | 40 |
| 9 | qw | 3 | 9 | 7 | 3 | 4 | 6 | 6 | 5.83 | 35 |
| 13 | qw | 0 | 0 | 0 | 3 | 4 | 0 | 8 | 2.5 | 15 |
| 18 | qw | 1 | 0 | 1 | 4 | 4 | 3 | 3 | 2.5 | 15 |
| 14 | tiw | 18 | 2 | 3 | 0 | 8 | 2 | 14 | 4.83 | 29 |
Serum IL-10 and IL-12p70
Cytokine levels over the course of the study are summarized in Table 2. We found lower serum IL-10 levels in non-responders (n=5) compared to responders (n=10) after treatment (pre-treatment p=0.80, overall post-treatment p<0.0001) (see Figure 1). There was an increase in serum IL-10/IL-12p70 ratio compared to baseline in the combined group of all patients on interferon-beta therapy (p=0.043). Within the group of non-responders, there was a decrease in serum IL-10/IL-12p70 ratio over the course of treatment (p=0.031). Serum IL-12p70 levels were lower in the qw group over the course of treatment, but not significantly different at baseline (pre-treatment p=0.15, post treatment p<0.0001).
Table 2(A).
Changes in Serum and Cellular Cytokine levels over 24 weeks of IFN-beta qw (n=8)
| pg/ml mean±SE | Pre-Rx mean | 4 weeks | 8 Weeks | 12 weeks | 16 Weeks | 20 weeks | 24 weeks | Post-Rx mean |
|---|---|---|---|---|---|---|---|---|
| Serum IL10 | 13.36±1.31 | 13.49±1.17 | 13.45±1.43 | 13.57±1.73 | 14.21±2.00 | 12.27±1.02 | 12.51±0.71 | 13.05±0.56 |
| Serum IL12p70 | 53.71±8.36 | 56.80±4.57 | 50.77±6.19 | 45.22±7.69 | 46.93±9.43 | 41.52±5.67 | 47.89±5.77 | 48.19±2.69* |
| Serum 10/12p70 | 0.253 ± 0.023 | 0.228 ± 0.0274 | 0.288 ± 0.044 | 0.329 ± 0.039 | 0.330 ± 0.035 | 0.318 ± 0.027 | 0.287 ± 0.035 | 0.297±0.014 |
| cellular IL10 | 424.8±125.5 | 330.3±59.06 | 336.2±100.4 | 1062±351.0 | 758.8±225.0 | 1249±553.7 | 547.6±111.7 | 713.9±123.1† |
| cellular IL12p40 | 1299±282.3 | 1266±450.3 | 1120±384.6 | 1381±321.6 | 1164±215.7 | 811.1±179.5 | 1028±177.6 | 1128±121.2 |
| cellular IL12p70 | 63.77±22.89 | 72.10±28.10 | 23.86±12.14 | 108.1±56.86 | 55.29±15.90 | 62.33±26.16 | 21.99±4.457 | 57.27±12.07 |
| cellular IFN-g | 845.8±261.2 | 417.1±307.0 | 239.2±97.97 | 221.4±54.02 | 194.5±67.42 | 299.5±104.4 | 341.2±154.0 | 285.5±61.21 |
†=value significantly different vs. pre-treatment (p<0.05 by repeated measures ANOVA)
*=value significantly different vs tiw (p<0.05 by Mann-Whitney)
Figure 1.
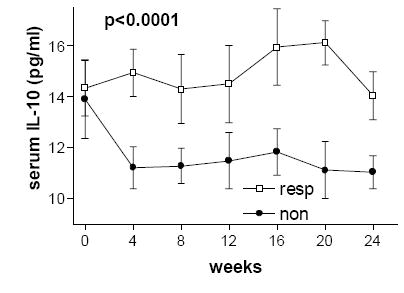
Serum levels of interleukin-10 were lower in non-responders (as defined by cumulative unique MRI lesions) over the course of 24 weeks of IFN-β therapy compared to non-responders. Standard error bars are shown.
Cellular IL-10, IL-12 and IFN-γ
Cytokine levels over the course of the study are summarized in Table 2. Cellular IL-10 levels increased over the course of treatment in the interferon beta qw group (p=0.0448, n=8) and in the combined groups on any interferon therapy (p=0.049, n=15). Cellular IL-10 levels were increased in both responders and non-responders to therapy without any significant difference based on MRI measures of response to therapy. There was an increase in cellular IL-12p70 levels over the course of treatment in the interferon tiw group (p=0.005, n=7), in the total interferon therapy group (p=0.0012, n=15) and within the groups of responders (p=0.014, n=10) and non-responders (p=0.04, n=5). We found lower cellular IL-12p70 levels in the 30 µg qw (n=8) as compared to the 44 µg tiw (n=7) group (p<0.0001). Cellular IL-12p70 levels were lower (p<0.02) in the group of non-responders versus responders (see Figure 2). There was a decrease in cellular stimulated IFN-γ levels over the course of therapy in the combined interferon-beta therapy groups (p<0.001, n=15). Cellular IFN-γ levels decreased over the course of treatment within the total group on any IFN therapy (p<0.001, n=15) and the group of responders (p=0.013, n=10) but not in the group of non-responders (p=0.12, n=5). Levels of cellular IFN-γ were lower in responders to therapy (pre-treatment p=0.18, post-treatment p=0.005) (see Figure 3). IFN-γ levels appeared to decrease over the course of therapy in both the qw and tiw treatment groups, but these results did not reach significance (p=0.068 and p=0.052, respectively).
Figure 2.
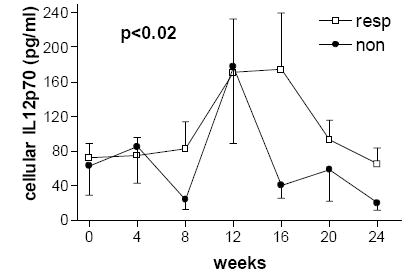
Levels of ex vivo cellular production of interleukin-12p70 over the course of 24 weeks of IFN-β therapy were lower in the group of non-responders compared to responders. Overlapping error bars are removed for clarity.
Figure 3.
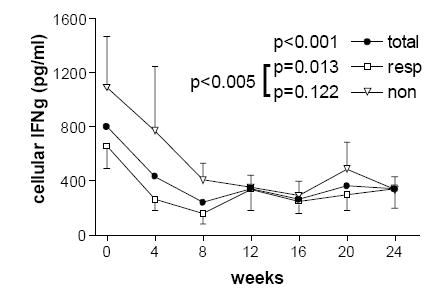
Levels of ex vivo cellular production of interferon-gamma decreased compared to baseline over the course of IFN-β therapy in the total group on any IFN-β therapy (p<0.001) and in the group of responders (p=0.013). Compared to non-responders, IFN-gamma levels were lower on treatment in responders (p=0.005). Overlapping error bars are removed for clarity.
4. Discussion
This is the first randomized, prospective clinical trial to directly compare the cytokine effects of different IFN-β-1a preparations in MS. Ours is the first study to demonstrate lower IL-10 levels in those patients with a poor radiologic response to initial IFN-β therapy compared to responders. Responders to IFN-β therapy had lower levels of cellular IFN-γ production compared to non-responders. We have also confirmed prior studies that IFN-β increases cellular production of IL-10 and IL-10/IL-12 ratio in MS.
IL-10 has multiple effects that may be beneficial in multiple sclerosis, including inhibition of T cell proliferation and macrophage IL-1 and TNF-α production (Imitola et al., 2005). IL-10 may also induce T cell anergy and regulatory T cell production (Groux et al., 1996, Roncarolo et al., 2002). Immature dendritic cells exposed to IL-10 resist LPS-induced maturation (Adikari et al., 2004). IL-10 also stimulates certain aspects of immune function, including macrophage phagocytosis and B cell proliferation and antibody production (Beebe et al., 2002). IL-10 reduces the severity of experimental autoimmune encephalomyelitis (EAE) depending on the route and timing of administration and IL-10 deficient mice develop more severe disease (Rott et al., 1994, Cua et al., 1999, Beebe et al., 2002, Bettelli et al., 1998). IL-10 decreases IL-12p35 and IL-12p40 mRNA expression in human monocytes (D’Andrea et al., 1993). We report for the first time that serum IL-10 levels are decreased in non-responders to IFN-β therapy. In contrast to other studies, we did not find a significant increase in serum IL-10 levels in response to IFN-β, though we did find an increase in cellular IL-10 levels in the qw IFN-β and combined IFN-β qw and tiw groups. We suspect this may be due to our smaller sample size which increases the chance of a type I error (null finding when a real difference exists). It is interesting that though IL-10 was decreased in non-responders in our study, ex vivo IL-12p70 levels were also decreased. Since IL-12 levels correlate with both clinical and MRI measures of disease severity, it is surprising that the group of non-responders actually had lower cellular IL-12p70 levels during therapy, suggesting that decreased IL-10 levels may have had greater influence (Makhlouf et al., 2001). However, serum IL-12p70 levels were not significantly different between responders and non-responders.
IL-12p40 mRNA increases at peak of EAE disease, while IL-12p35 remains unchanged (Jander and Stoll 1998). IL-12 administration worsens EAE, and IL-12 deficient mice are resistant to EAE induction despite increased IFN-γ levels (Smith et al., 1997, Segal et al., 1996). IL-12 deficient mice regain susceptibility to disease induction if the inoculated autoreactive cells are primed with IL-12 (Segal et al., 1996). IL-12 administration worsens EAE even in the presence of anti-IFN-γ, suggesting the mechanisms of IL-12 in EAE are not necessarily dependent on IFN-γ (Leonard et al., 1995). IL-12p40 mRNA is increased before disease relapse in humans and IL-12 levels correlate with disease activity (Windhagen et al., 1995, Van Boxel-Dezaire et al., 1999, Makhlouf et al., 2001). Our study did not find any significant differences in cellular PBMC IL-12p40 production. We did find that cellular IL-12p70 levels were lower within the group of non-responders compared to responders to therapy, a finding not replicated by serum IL-12p70 levels. The only significant differences we found between qw and tiw therapy were lower serum and cellular IL-12p70 levels in the qw group during treatment.
Given the potential benefits of increased IL-10 and potential harm of IL-12, several groups have reported improvements in the ratio of IL-10 to IL-12 with IFN-β treatment (Correale et al., 1995, Rudick et al., 1996, Wang et al., 2000, Byrnes et al., 2002, Berghella et al., 2005, Ersoy et al., 2005). We confirmed these prior reports of overall improved ratios of serum IL-10/IL-12p70 with IFN-β treatment and found that ratios of serum IL-10/IL-12p70 decreased in non-responders to therapy compared to pre-treatment.
Data on the effects of IFN-γ in MS and EAE have provided conflicting results. IFN-γ activity is increased before and during EAE and decreased during disease remission, however IFN-γ knockout mice exhibit more severe disease (Imitola et al., 2005). In MS, relapses are associated with increased IFN-γ and IFN-γ administration precipitated relapses (Beck et al., 1988, Panitch et al., 1987, Becker et al., 1999). We here confirm prior studies that IFN-β therapy decreases IFN-γ in MS (Becher et al., 1999, Furlan et al., 2000, Berghella et al., 2005). We also found that IFN-β administration decreases ex vivo stimulated IFN-γ production by PBMC in responders to IFN-β. Non-responders to IFN-β did not significantly suppress IFN-γ production compared to baseline, but this null finding may be due to a type I error due to the small sample size of the non-responder group. Post-treatment IFN-γ levels were lower in responders to therapy compared to non-responders. Within the qw and tiw groups, there was a decrease in cellular IFN-γ levels but this finding did not reach statistical significance.
Prior studies have found that lower baseline levels of cellular IFN-γ and IL-12p35 mRNA predict clinical responders to IFN-β therapy (Van Boxel-Dezaire et al., 2000, Petereit et al., 2002). Baseline levels of cellular IFN-γ were lower in responders in our study but not significantly different from non-responders. An IL-10 promoter haplotype has also been shown to predict response to IFN-β therapy (Wergeland et al., 2005). In addition, we have previously reported that tiw IFN-β-1a induced increased serum soluble vascular cell adhesion molecule (sVCAM) levels compared to qw IFN-β-1a, and that sVCAM levels correlated with a decreased number of MRI lesions (Graber et al., 2005). Given the findings of these studies and our current report along with the considerable heterogeneity of multiple sclerosis, it is conceivable that the different cytokine changes induced by different IFN-β preparations might make one treatment more effective than another for a particular patient or guide changes to other modes of therapy.
Limitations of this study include the small sample size and short follow-up time, with a limited power to detect small differences given the heterogeneous nature of cytokine levels and disease activity in MS between individuals. As for the length of our study, it has been shown that IFN-β treatment induces significant differences in MRI measures of disease activity within the time frame of our follow-up (Calabresi et al., 1997). It has previously been reported that IL-10 levels are decreased before relapse, but the number of clinical relapses in our group of non-responders was too small to influence the overall measures of IL-10.
In summary, we have conducted the first prospective randomized trial comparing cytokine effects of different IFN-β-1a preparations in relation to radiologic response to therapy. We have confirmed prior reports by others that IFN-γ levels are reduced in response to IFN-β treatment. We also confirmed prior reports that IFN-β therapy increases the ratio of serum IL-10 to serum IL12p70, but found a decreased serum IL10/IL12p70 ratio in non-responders. Interestingly, we found that cellular stimulated IL12p70 levels were actually increased during IFN-β therapy in responders to treatment but not in non-responders. We also found that cellular IL12p70 levels were lower in non-responders to therapy. We report for the first time that serum IL10 levels are decreased in non-responders to IFN-β therapy.
Table 2(B).
Changes in Serum and Cellular Cytokine levels over 24 weeks of IFN-beta tiw (n=7)
| pg/ml mean±SE | Pre-Rx mean | 4 weeks | 8 weeks | 12 weeks | 16 weeks | 20 weeks | 24 weeks | Post-Rx mean |
|---|---|---|---|---|---|---|---|---|
| Serum IL10 | 15.14±1.08 | 14.40±0.84 | 13.55±1.45 | 15.73±1.56 | 14.72±1.39 | 13.91±1.16 | 14.19±1.61 | 14.42±0.53 |
| Serum IL12p70 | 76.15±9.05 | 80.75±15.48 | 88.92±15.60 | 75.71±10.41 | 70.62±13.11 | 64.17±13.78 | 73.31±13.62 | 75.58±5.41* |
| Serum 10/12p70 | 0.208 ± 0.027 | 0.217 ± 0.038 | 0.178 ± 0.030 | 0.220 ± 0.021 | 0.260 ± 0.047 | 0.276 ± 0.063 | 0.235 ± 0.050 | 0.231±0.017 |
| cellular IL10 | 360.7±121.8 | 427.6±220.1 | 412.8±155.0 | 725.5±149.7 | 1499±600.2 | 1246±594.4 | 1648±992.3 | 993.1±223.0 |
| cellular IL12p40 | 2146±548.1 | 1602±282.6 | 1168±308.1 | 2125±508.1 | 1753±345.9 | 1635±317.8 | 1033±136.5 | 1578±141.3 |
| cellular IL12p70 | 75.21±20.85 | 84.84±26.67 | 106.9±40.22 | 247.3±76.49 | 214.7±89.97 | 102.5±29.26 | 81.64±23.72 | 139.7±23.18† |
| cellular IFN-g | 755.7±222.3 | 451.9±131.4 | 244.8±111.7 | 482.2±214.0 | 341.8±127.7 | 434.3±187.7 | 341.9±139.6 | 382.8±61.12 |
†=value significantly different vs. pre-treatment (p<0.05 by repeated measures ANOVA)
*=value significantly different vs qw (p<0.05 by Mann-Whitney)
Table 2(C).
Changes in Serum and Cellular Cytokine levels over 24 weeks of Any Interferon Therapy (n=15)
| pg/ml mean±SE | Pre-Rx mean | 4 weeks | 8 weeks | 12 weeks | 16 weeks | 20 weeks | 24 weeks | Post-Rx mean |
|---|---|---|---|---|---|---|---|---|
| Serum IL10 | 14.19±0.86 | 13.29±0.76 | 13.49±0.98 | 14.58±1.17 | 14.45±1.21 | 13.04±0.77 | 13.29±0.84 | 13.63±0.35 |
| Serum IL12p70 | 64.18±6.63 | 67.98±7.98 | 68.58±9.21 | 59.45±7.35 | 57.98±8.25 | 52.09±7.46 | 59.75±7.58 | 60.97±3.23 |
| Serum 10/12p70 | 0.232±0.018 | 0.223±0.022 | 0.236±0.030 | 0.278±0.027 | 0.297±0.029 | 0.298±0.032 | 0.263±0.030 | 0.266±0.012† |
| cellular IL10 | 394.9±85.1 | 375.7±103.9 | 371.9±87.2 | 905.0±198.4 | 1104±308.7 | 1247±390.4 | 1061±470.9 | 844.2±123.2† |
| cellular IL12p40 | 1694±306.7 | 1423±268.4 | 1142±241.8 | 1728±298.4 | 1439±206.2 | 1196±202.1 | 1031±110.2 | 1379±92.0 |
| cellular IL12p70 | 69.1±15.1 | 78.0±18.9 | 62.6±22.0 | 173.1±48.8 | 129.7±46.2 | 81.1±19.6 | 49.8±13.5 | 95.7±13.3† |
| cellular IFN-g | 803.8±168.0 | 433.3±169.2 | 241.8±71.1 | 343.1±105.6 | 263.3±69.7 | 362.4±101.4 | 341.5±101.1 | 330.9±43.4† |
†=value significantly different vs. pre-treatment (p<0.05 by repeated measures ANOVA)
Acknowledgments
This work was supported in part by USPHS training grant HL07612-15 (Dr. Graber), Serono, Inc., and The National Institute of Neurological Disorders and Stroke grant K-24-NS02082 (Dr. Dhib-Jalbut). The authors wish to thank Ms. Rameeza Allie, Dr. Tapas Makar, and Dr. Peter A. Calabresi for their helpful advice and technical support during the course of this project. The authors wish to thank Dr. Jordan Warnick and the Office of Student Research of the University of Maryland at Baltimore School of Medicine for supporting this research. In addition, the authors would also like to sincerely thank the individuals who volunteered to be subjects in this clinical trial, without whom this work could never have been conducted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adikari SB, Pettersson A, Soderstrom M, Huang YM, Link H. Interleukin-10-modulated immature dendritic cells control the proinflammatory environment in multiple sclerosis. Scand J Immunol. 2004;59:600–606. doi: 10.1111/j.1365-3083.2004.01453.x. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- Becher B, Giacomini PS, Pelletier D, McCrea E, Prat A, Antel JP. Interferon-gamma secretion by peripheral blood T-cell subsets in multiple sclerosis: correlation with disease phase and interferon-beta therapy. Ann Neurol. 1999;45:247–250. [PubMed] [Google Scholar]
- Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger exacerbations? Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS) Cytokine and Growth Factor Reviews. 2002;12:403–412. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Berghella AM, Totaro R, Pellegrini P, Contasta I, Russo T, Carolei A, et al. Immunological study of IFNβ-1a-treated and untreated multiple sclerosis patients: clarifying IFNβ mechanisms and establishing specific dendritic cell immunotherapy. Neuroimmunomodulation. 2005;12:29–44. doi: 10.1159/000082362. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Byrnes AA, MacArthur JC, Karp CL. Interferon-beta therapy for multiple sclerosis induces reciprocal changes in interleukin-12 and interleukin-10 production. Ann Neurol. 2002;51:165–174. doi: 10.1002/ana.10084. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Stone LA, Bash CN, Frank JA, McFarland HF. Interferon beta results in immediate reduction of contrast-enhanced MRI lesions in multiple sclerosis patients followed by weekly MRI. Neurology. 1997;48:1446–1448. doi: 10.1212/wnl.48.5.1446. [DOI] [PubMed] [Google Scholar]
- Correale J, Gilmore W, MacMillan M, Li S, McCarthy K, Le T, et al. Patterns of cytokine secretion by proteolipid protein-specific T cell clones during the course of multiple sclerosis. J Immunol. 1995;154:2959–2968. [PubMed] [Google Scholar]
- Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interluekin 10 (IL10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, Independent Comparison of Interferon (INCOMIN) trial study group Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomized multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- Ersoy E, Kus CNS, Sener U, Coker I, Zorlu Y. The effects of interferon-beta on interleukin-10 in multiple sclerosis patients. Eur J Neurol. 2005;12:208–211. doi: 10.1111/j.1468-1331.2004.00986.x. [DOI] [PubMed] [Google Scholar]
- Furlan R, Bergami A, Lang R, Brambilla E, Franciotta D, Martinelli V, et al. Interferon-β treatment in multiple sclerosis patients decreases the number of circulating T cells producing interferon-γ and interleukin-4. J Neuroimmunol. 2000;111:86. doi: 10.1016/s0165-5728(00)00377-5. [DOI] [PubMed] [Google Scholar]
- Graber J, Zhan M, Ford D, Kursch F, Francis G, Bever C, Panitch H, Dhib-Jalbut S. Interferon-beta-1a induces increases in soluble vascular cell adhesion molecule: implications for its mode of action in multiple sclerosis. J Neuroimmunol. 2005;161:169–176. doi: 10.1016/j.jneuroim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacology and Therapeutics. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Interferon Beta Multiple Sclerosis Study Group. Interferon-beta-1b is effective in relapsing-remitting multiple sclerosis: I. clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- Jander S, Stoll G. Differential induction of interleukin-12, interleukin-18 and interleukin-1β converting enzyme mRNA in experimental autoimmune encephalomyelitis of the Lewis rat. J Neuroimmunol. 1998;91:93–99. doi: 10.1016/s0165-5728(98)00162-3. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Waldburger KE, Goldman SJ. Regulation of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Janeway CA., Jr Interferon-gamma plays a critical role in induced cell death of effector T cells: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luomala M, Lehtimaki T, Huhtala H, Ukkonen M, Koivula T, Hurme M, et al. Promoter polymorphism of IL-10 and severity of multiple sclerosis. Acta Neurol Scand. 2003;108:396–400. doi: 10.1034/j.1600-0404.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- Makhlouf K, Weiner H, Khoury S. Increased percentage of of IL-12+ monocytes in the blood correlates with the presence of active MRI lesions in MS J Neuroimmunol. 2001;119:145–149. doi: 10.1016/s0165-5728(01)00371-x. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Goodin DS, Francis G, Chang P, Coyle PK, O’Conner P, EVIDENCE Study Group Evidence of interferon dose response: European North American Comparative Efficacy. University of British Columbia MS/MRI Research Group. Randomized comparative study of of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology. 2002;59:1507–1517. doi: 10.1212/01.wnl.0000034080.43681.da. [DOI] [PubMed] [Google Scholar]
- Paty DW, Li DK, Interferon Beta Multiple Sclerosis Study Group Interferon-beta-1b is effective in relapsing-remitting multiple sclerosis: II. MRI analysis of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol. 2000;165:1641–1651. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- Petereit HF, Nolden S, Schoppe S, Bamborschke S, Pukrop R, Heiss WD. Low interferon gamma producers are better treatment responders: a two-year follow-up of interferon beta-treated multiple sclerosis patients. Mult Scler. 2002;8:492–494. doi: 10.1191/1352458502ms853oa. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and Disability by Interferon-beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Cytokine mRNA levels in mononuclear blood cells from patient with multiple sclerosis. Neurology. 1994;44:1523–1526. doi: 10.1212/wnl.44.8.1523. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bachetta R, Bordignon C, Narula S, Levings MK. Type 1 regulatory cells. Immunol Rev. 2002;182:68–80. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Rott O, Fliescher B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Ransahoff RM, Peppler R, Vanderbrug MS, Lehmann P, Alam J. Interferon beta induces interluekin-10 expression: relevance to multiple sclerosis. Ann Neurol. 1996;40:618–627. doi: 10.1002/ana.410400412. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Ransahoff R, Lee J, Peppler R, Yu M, Mathisen P, et al. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology. 1998;50:1294–1300. doi: 10.1212/wnl.50.5.1294. [DOI] [PubMed] [Google Scholar]
- Sega S, Wraber B, Mesec A, Horvat A, Ihan A. IFN-β1a and IFN-β1b have different patterns of influence on cytokines. Clinical Neurology and Neurosurgery. 2004;106:255–258. doi: 10.1016/j.clineuro.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184:771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JH, Jacobs LD, Campion MK, Rudick RA, Cookfair DL, Herndon RM, et al. The Multiple Sclerosis Collaborative Research Group (MSCRG) A longitudinal study of brain atrophy in relapsing multiple sclerosis. Neurology. 1999;53:139–148. doi: 10.1212/wnl.53.1.139. [DOI] [PubMed] [Google Scholar]
- Smith T, Hewson AK, Kingsley CI, Leonard JP, Cuzner ML. Interleukin-12 induces relapse in experimental allergic encephalomyelitis in the Lewis rat. Am J Pathol. 1997;150:1909–1917. [PMC free article] [PubMed] [Google Scholar]
- Van Boxel-Dezaire AH, Hoff SC, Van Oosten BW, Verweij CL, Drager AM, Ader HJ, et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Van Boxel-Dezaire AH, Van Trigt-Hoff S, Killestien J, Schrijver H, Van Houwelingen J, Polman C, et al. Contrasting responses to interferon beta-1b treatment in relapsing-remitting multiple sclerosis: does baseline interleukin-12p35 messenger RNA predict the efficacy of treatment? Ann Neurol. 2000;48:313–322. doi: 10.1002/1531-8249(200009)48:3<313::aid-ana5>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen M, Wandinger KP, William G, Dhib-Jalbut S. IFN-beta-1b inhibits IL-12 production in peripheral blood mononuclear cells in an IL-10-dependent mechanism: relevance to IFN-beta-1b therapeutic effects in multiple sclerosis. J Immunol. 2000;165:548–557. doi: 10.4049/jimmunol.165.1.548. [DOI] [PubMed] [Google Scholar]
- Waubant E, Fee L, Bachetti P, Sloan R, Cotleur A, Rudick R, et al. Relationship between serum levels of IL-10, MRI activity and interferon beta-1a therapy in patients with relapsing remitting MS. J Neuroimmunol. 2001;112:139–145. doi: 10.1016/s0165-5728(00)00355-6. [DOI] [PubMed] [Google Scholar]
- Wergeland S, Beiske A, Nyland H, Hovdal H, Jensen D, Larsen JP, et al. IL-10 promoter haplotype influence on interferon treatment response in multiple sclerosis. Eur J Neurol. 2005;12:171–175. doi: 10.1111/j.1468-1331.2004.01102.x. [DOI] [PubMed] [Google Scholar]
- Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, et al. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


