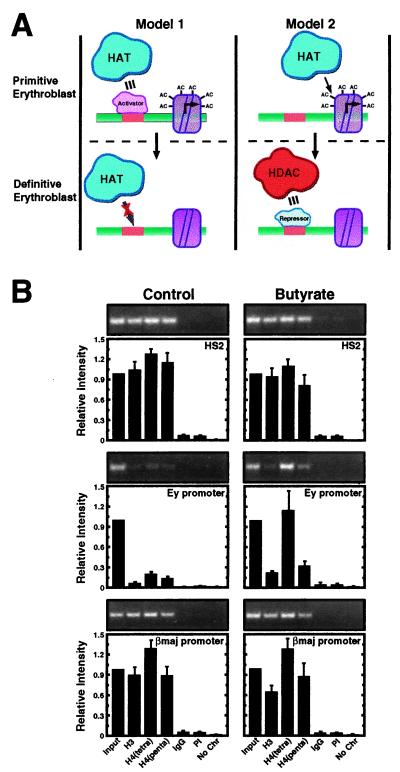
Figure 6.
HDAC inhibition selectively induces acetylation at the developmentally silenced Ey promoter. (A) Models to explain the hypoacetylated state of the embryonic β-globin promoters in 14.5 dpc fetal liver. Model 1 assumes that defective HAT recruitment in the definitive erythroblast is responsible for hypoacetylation. The defect would result from limiting amounts of an activator required to recruit HATs to the promoters. Model 2 assumes that a stage-specific repressor recruits HDACs to the embryonic β-globin promoters in definitive erythroblasts and therefore establishes the hypoacetylated state. HATs recruited by the LCR would be sufficient to generate a hyperacetylated state in primitive erythroblasts lacking the repressor. (B) Fetal liver cells (14.5 dpc) were incubated with or without butyrate for 4 h, and then the acetylation state of the endogenous murine β-globin locus was measured by ChIP analysis. Representative ethidium bromide-stained gels are shown. The graphs show the relative intensity of the PCR products from three independent experiments (mean ± SEM). Note that butyrate treatment increased the acetylation state only of the Ey promoter.