Figure 2.
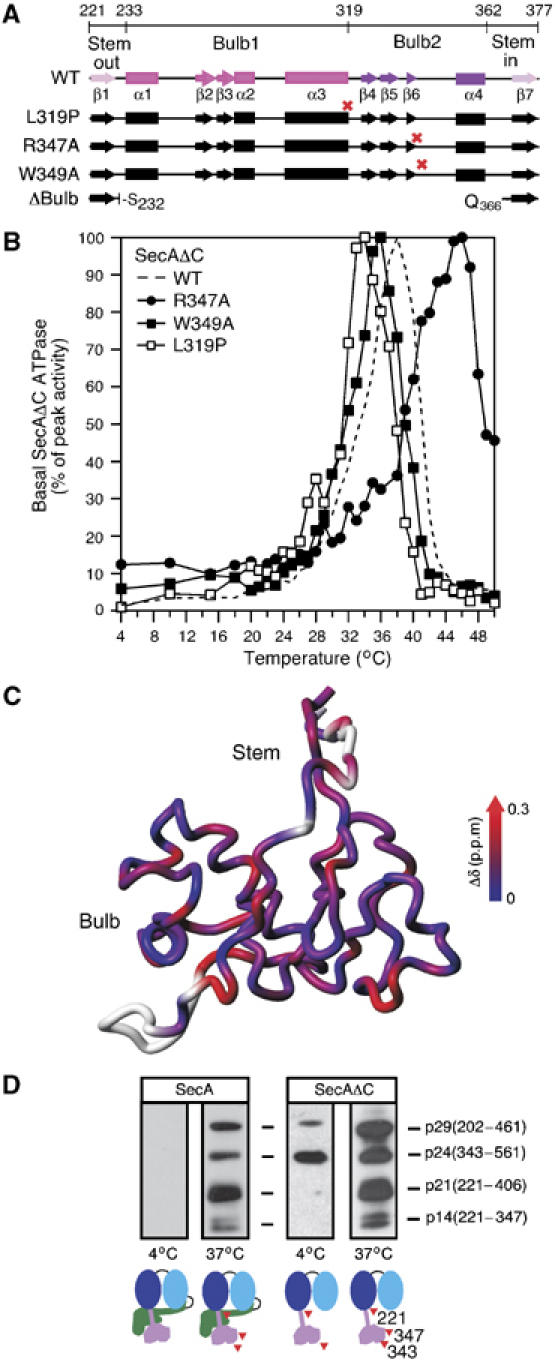
(A) PBD deletions, point mutations (red crosses) and secondary structure are indicated on a region map. β1 and β7 (pink) together form the antiparallel sheet of the Stem. Between them lie the two globular halves of the Bulb (1, magenta and 2, purple). See also Figure 1A. (B) Basal ATPase activities of SecAΔC, SecAΔC(R347A), SecAΔC(W349A) and SecAΔC(L319P) (as in Figure 1D). (C) Chemical shift change (Δδ) as a function of temperature mapped onto the structure of PBD. Higher Δδ values indicate temperature-induced unfolding. (D) SecA or SecAΔC (245 pmol in 100 μl buffer B) were treated (13 min) by limited trypsinolysis at 4°C (0.25 μg/ml) or 37°C (0.025 μg/ml). Trypsin was inactivated with Pefabloc (9 mM). Following SDS–PAGE, polypeptides were immunostained by α-PBD-specific antibody and were identified by N-terminal sequencing.
