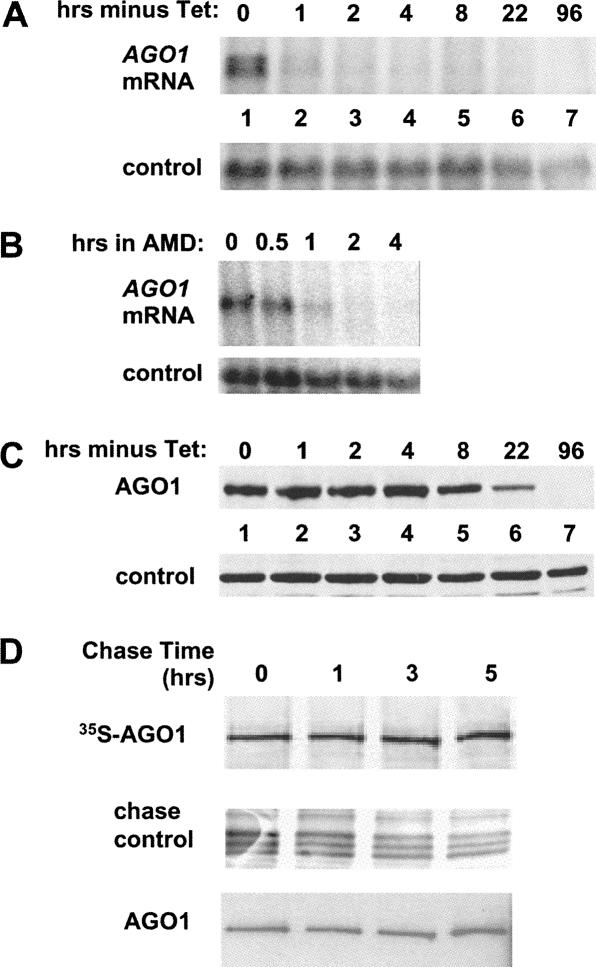
FIGURE 1.
(A) Time course of TbAGO1 mRNA disappearance after removal of tet. TbAGO1KOc cells were washed twice in medium and then put back in culture in tet-minus medium for the times indicated above each lane. RNA was extracted and analyzed by Northern blot using a TbAGO1 coding-region probe. Note that two hybridizing bands are seen in lane 1; they are derived from utilization of two alternate 3′-splice sites present upstream of the TbAGO1 coding region. Control: the membrane was stripped and rehybridized with a probe detecting the mitochondrial large ribosomal RNA. (B) Decay of TbAGO1 mRNA in wild-type trypanosomes. Wild-type trypanosomes were incubated with the transcription inhibitor actinomycin D (AMD) as previously described (Shi et al. 2004b). RNA samples were prepared at the times indicated above each lane and analyzed by Northern blotting with an AGO1 coding-region probe. Control: hybridization to the 18S rRNA as a loading control. (C) Time course of the disappearance of TbAGO1 after tet removal. TbAGO1KOc cells were washed and incubated in tet-free medium as described in the legend of A. Whole-cell extracts were fractionated on a 6% SDS-PAGE and processed for Western blotting with anti-AGO1 antibodies (Shi et al. 2004b). Control indicates a cross-reactive protein. (D) Endogenous TbAGO1 is a stable protein. A cell line expressing BB2-TAP-tagged TbAGO1 (Shi et al. 2004b) was metabolically labeled with 35S-methionine as described in Materials and Methods. After a 2-h pulse, the label was chased with an excess of unlabeled methionine, and cytoplasmic extracts were prepared at the times indicated above each lane. TbAGO1 was then immunoprecipitated with anti-BB2 antibodies, the immunoprecipitates were separated by SDS-PAGE, and the radioactive bands were detected by autoradiography. The panel chase control shows a group of bands from the total protein samples run in parallel to show that the chase was effective. As a control for the efficacy of the immunoprecipitation, the membrane shown in the upper panel was analyzed by Western blotting with the anti-BB2 antibody, which detects the total amount of AGO1 protein present in the immunoprecipitates.