Figure 1.
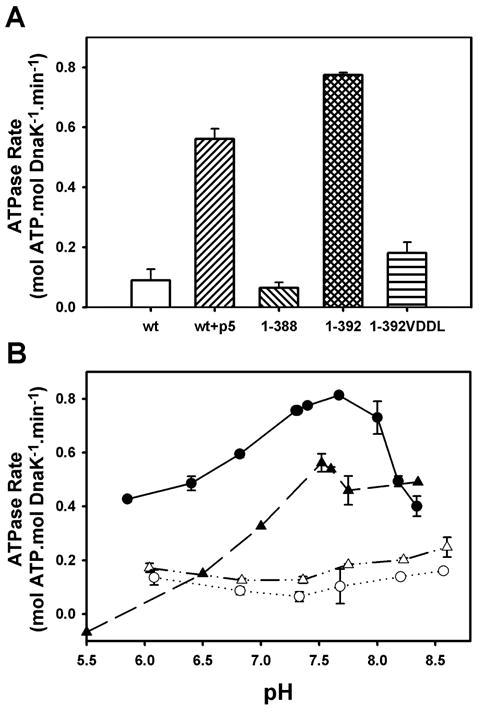
The linker stimulates the ATPase activity of the isolated ATPase domain, similar to the effect of substrate on full-length DnaK. (A) Steady-state ATPase rates measured at pH 7.6. (B) pH-dependence of steady-state ATPase activities for full-length wild-type DnaK in the absence (△) and presence (▲) of p5 peptide, DnaK(1-388) (○), and DnaK(1-392) (●). Error bars represent standard deviation from ≥3 experiments.
