Figure 6.
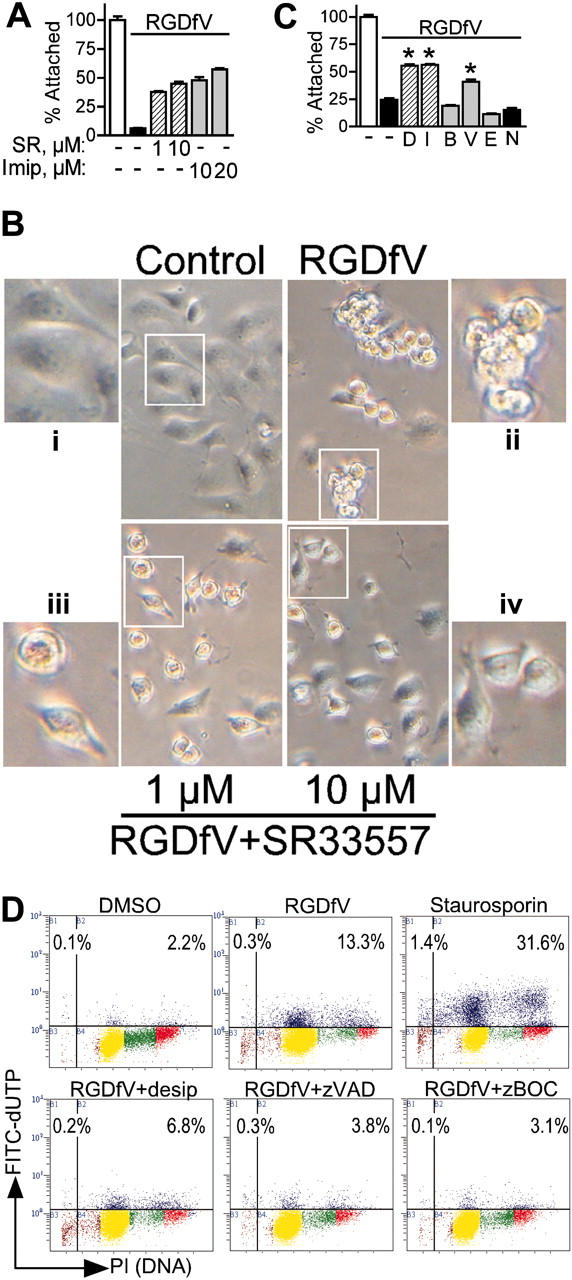
Inhibitors of acid sphingomyelinase prevent HBMEC detachment and loss of cell spreading that is induced by αvβ3/αvβ5-integrin inhibition. (A) HBMECs (4 × 104 cells/well) were allowed to adhere and spread in 48-well non–tissue culture plates coated with vitronectin and blocked with BSA. SR33557 ( ), imipramine (
), imipramine ( ), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM,
), or vehicle control (▪) was added 2 hours after plating, followed 2 hours later with RGDfV (25 mg/mL) for an additional 18 hours. Detached cells were washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (□, 100%). P < .001 for RGDfV with SR33557 or imipramine compared with RGDfV alone, by unpaired t tests; n = 8 for each condition. (B) HBMECs (2 × 105 cells/well) were allowed to adhere and spread for 2 hours in 6-well plate coated with vitronectin and blocked with BSA. SR33557 (1 or 10 μM; iii-iv) or vehicle (i-ii) was added, followed 2 hours later by RGDfV (25 μg/mL; ii-iv) or vehicle (i). Cells were photographed 18 hours later, as in Figure 1C. Insets depict enlargement of white rectangle in the adjacent figure. Original magnification, × 400. (C) HBMECs (4 × 104 cells/well) were allowed to adhere and spread on plates coated with 10 μM vitronectin and blocked with BSA. After 2 hours, desipramine (D; 10 μM,  ), imipramine (I; 10 μM,
), imipramine (I; 10 μM,  ), Z-VAD-FMK (V; 25 μM,
), Z-VAD-FMK (V; 25 μM,  ), BOC-D-FMK (B; 25 μM,
), BOC-D-FMK (B; 25 μM,  ), Z-DEVD-FMK (E; 100 μM,
), Z-DEVD-FMK (E; 100 μM,  ), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.
), or control caspase inhibitor (N, ▪), or vehicle control (-; ▪), was added to the medium, followed 2 hours later with RGDfV (25 mg/mL, indicated by horizontal bar) or vehicle (DMSO, □) for an additional 18 hours. Detached cells were then washed off and remaining cells were detected by MTT. Optical density was normalized compared with cells treated with vehicle control (clear bar, 100%). * denotes P < .001 (for RGDfV with desipramine, imipramine, or Z-VAD-FMK, compared with vehicle, by unpaired t tests [*]; n = 6-8 for each condition). (D) HBMECs (106 cells/10-cm dish) were allowed to spread on VN blocked with BSA. Then, 2 hours later, desipramine (10 μM) or the pan-caspase inhibitors Z-VAD-FMK or BOC-D-FMK (25 μM) was added. Following another 2 hours, RGDfV (25 μg/mL) or vehicle control (DMSO) was added for additional 18 hours. Cells treated with staurosporin (500 nM) served as positive control. Apoptosis was assessed by flow cytometry using the Apo-Direct kit. Percent values on the figure indicate percentage of cells with DNA damage in the 2 top quadrants (blue). FITC-dUTP indicates fluorescein isothiocyanate–deoxyuridine triphosphate.
