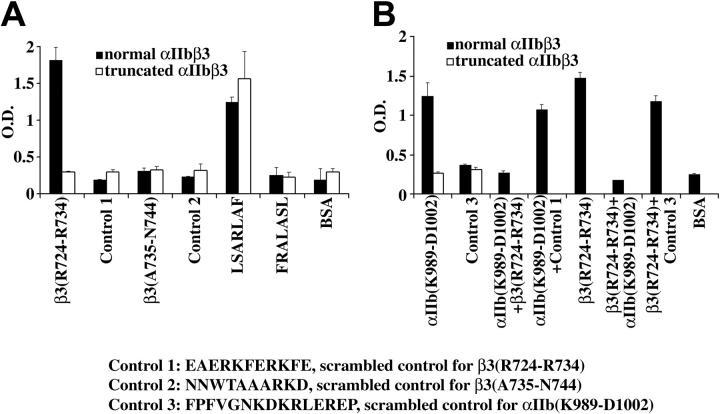
Figure 6.
The β3 cytoplasmic domain peptide RKEFAKFEEER binds to the cytoplasmic domain of αIIb. The details of the methodology for the binding experiments are described in “Patient, materials, methods.” (A) Normal αIIbβ3 bound to the peptides RKEFAKFEEER and LSARLAF, but not to the β3 peptide ARAKWDTANN, or the scrambled control peptides EAERKFERKFE, NNWTAAARKD, and FRALASL. In contrast, truncated αIIbβ3 did not bind to RKEFAKFEEER; it bound only to LSARLAF. (B) Normal αIIbβ3 bound to the αIIb cytoplasmic domain peptide KVGFFKRNRPPLEED but not to the scrambled version FPFVGNKDKRLEREP. Truncated αIIbβ3 did not bind to KVGFFKRNRPPLEED or FPFVGNKDKRLEREP. The β3 peptide RKEFAKFEEER but not its scrambled control version (Control 1) inhibited the binding of αIIbβ3 to immobilized KVGFFKRNRPPLEED. Conversely, KVGFFKRNRPPLEED, but not the scrambled control version (Control 3) inhibited the binding of αIIbβ3 to RKEFAKFEEER. The simplest interpretation of these data is that RKEFAKFEEER or a sequence therein can bind to the cytoplasmic domain of αIIb. The data represent the results of 3 experiments. The error bars represent SD, n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.