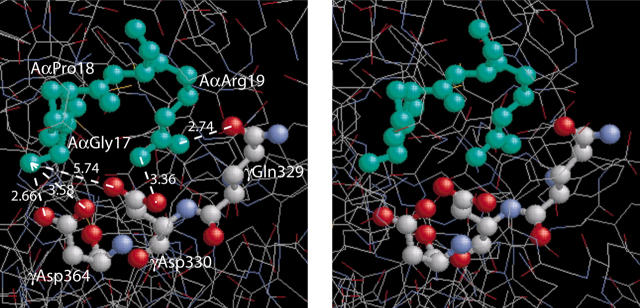
Figure 6.
A stereo figure showing the residues GPR, corresponding to the fibrin α-chain N-terminus, bound to the polymerization pocket in the globular γ-chain portion of fragment D19 and depicting the actual bond lengths (Å). The GPR sequence of the GPRPam peptide mimics the working part of A-knob composed of the amino acid residues (Aα17-19) exposed after cleavage of FpA. The a-hole is represented by 3 selected amino acid residues (γ329, γ330, and γ364) that are directly involved in the interaction.1,9 Considering GPRP the “surrogate A-knob”17 it is likely that the driving force of the A:a binding are the multiple electrostatic and hydrogen bonds between the α-amino group of AαGly17 and the guanidinium group of AαArg19 of an A-knob, on the one hand, and γAsp330 and γAsp364 along with carboxyamide of γGln329 of an a-hole, on the other hand. This aggregate of bonds stabilizing the A:a coupling is abruptly broken as soon as the knob and the hole are pulled apart to a distance of about 3 Å.