Figure 2.
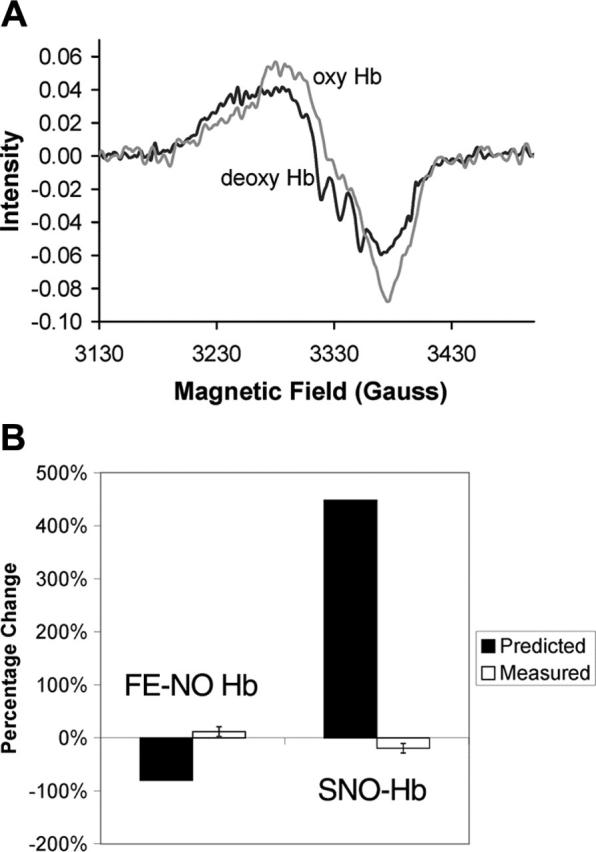
Lack of allosterically controlled intramolecular transfer. (A) Typical EPR spectra of Hb before and after oxygenation. The triplet hyperfine structure characteristic of pentacoordinate α-nitrosyl Hb present in the deoxygenated sample (deoxy Hb) disappears upon oxygenation (oxyHb). For this sample, the total Hb concentration was 4.5 mM and was 99.6% deoxygenated when ProliNO was added. Before oxygenation the total Fe-NO Hb was 3.8 μM, of which 53% was on the β subunits and 24% was of the pentacoordinate α-nitrosyl form. After oxygenation, the total Fe-NO Hb was measured to be 4.1 μM, of which 47% was on the β subunits and 0% was of the pentacoordinate α-nitrosyl form. (B) Measured changes in Fe-NO Hb and SNO-Hb versus those predicted by the SNO-Hb hypothesis. The predictions are shown assuming 80% transfer from heme to thiol. Thus, the prediction is that the amount of Fe-NO Hb would go from 3.7 μM to 0.74 μM upon oxygenation, giving a percentage change of 2.96/3.7 = 80%. The SNO-Hb is predicted to go from 0.66 μM to 3.62 μM (2.96 μM is transferred from the heme iron), giving a percentage change of 2.96/0.66 = 448%. These predictions are based on data presented by Gow and Stamler that measured the amount of SNO-Hb yield as a function of the NO/Hb ratio.2 Below a NO/Hb ratio of 1, as the NO/Hb ratio increased, the SNO-Hb yield decreased.2 At the lowest NO/Hb ratio tested of 0.01, the reported yield was 80%.2 Our samples had an NO/Hb ratio below 0.001. Error bars shown in measured values correspond to 1 standard deviation.
