A common point mutation in a conserved negative regulatory domain of the JAK2 (Janus kinase 2) tyrosine kinase leads to constitutive hematopoietic growth factor receptor signaling and was recently described in many patients with myeloproliferative disorders (MPDs), especially polycythemia vera.1-4 However, this JAK2 mutation is present in only a subset (35%-50%) of patients with myelofibrosis with myeloid metaplasia (MMM) or essential thrombocythemia, and it is rare in atypical MPDs, myelodysplastic syndrome, or other neoplastic myeloid disorders.5-7 Even when present, the JAK2 mutation is associated with phenotypic diversity. Cooperating mutations or distinct genetic/cellular background must be responsible for the variety of myeloid neoplasms associated with JAK2, yet such mutations have not yet been described, and the proliferative signals responsible for MPDs in the absence of JAK2 mutations remain largely unknown.
We read with great interest the recent reports8,9 of mice with homozygous point mutations in either the DNA binding domain or leucine zipper domain of the c-Myb (v-avian myeloblastosis virus oncogene homolog) transcription factor. These mice develop thrombocytosis on either thrombopoietin (TPO) receptor–deficient or wild-type backgrounds, as do patients with MMM. An eerily similar phenotype arises in mice with homozygous germ-line point mutations in the c-Myb binding surface (KIX domain) of the p300 transcription coactivator.10 The key role of c-Myb and p300 in normal hematopoiesis is also demonstrated by embryonic lethality of murine knock-out models due to failure of definitive hematopoiesis,11 as well as the apparent requirement for c-Myb down-regulation for normal thrombocyte development.12 In both p300 and c-Myb mutant mice, disordered megakaryocytopoiesis leads to marrow and spleen hypercellularity, peripheral blood thrombocytosis independent of thrombopoietin signaling, erythroid hypoplasia and anemia, and various abnormalities of lymphocyte development—all prominent characteristics in some forms of human MPDs, especially MMM. Therefore, MYB and EP300 (E1A binding protein p300)—the human homologs of genes encoding murine c-Myb and p300, respectively—are attractive candidate genes for human MPD.
After study approval by the Mayo Clinic institutional review board, we analyzed peripheral blood granulocyte genomic DNA from 25 patients with cellular or fibrotic MMM for mutations in the coding region of MYB (all 15 exons) and analyzed 30 additional patients (10 MMM, 10 with essential thrombocythemia, and 10 with polycythemia vera) for mutations in the region of EP300 that encodes the KIX domain. All 55 patients fit established World Health Organization diagnostic criteria and gave written informed consent for molecular analysis of blood samples. We used polymerase chain reaction (PCR) and standard automated fluorescent dye chemistry DNA sequencing (primers and amplification protocols available from the authors on request), supplemented by denaturing high-performance liquid chromatography to detect mutations with mixed clonality. The analysis was complemented by expression analysis by real-time PCR where mRNA was available.
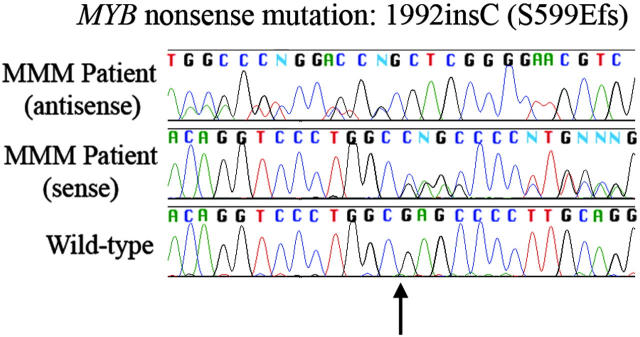
EP300 was unremarkable in all 30 patients tested. A single MYB heterozygous/mixed clonality nonsense mutation (c.1992insC and protein [p].S599EfsX605; GenBank reference sequences NM_005375 and NP_005366, respectively)16 was found in a fibrotic MMM sample (Figure 1). Buccal cells were not available from this patient to confirm whether the mutation was somatic or germ line; regardless, it may not be pathogenic, as the leucine zipper, interaction, and regulatory domains are all N-terminal— there are normal Myb isoforms in which all residues C-terminal to 351 (SwissProt isoform 3) are truncated and others in which residues carboxy-terminal to 567 are altered (isoform 6). The change was not present in 25 healthy controls (Continental European, Icelandic, and African-Jamaican DNA). We also detected a few synonymous single nucleotide polymorphisms in MYB, all also present in samples from healthy persons. Expression of MYB at the mRNA level did not differ significantly between MPD patients and healthy or anemic (non-MPD) controls. We conclude that point mutations in the coding region of MYB and P300 may cause murine myeloproliferation, but are probably not common in human classical MPDs. While the c-Myb and p300 mutant mice appear to be excellent systems for studying mammalian hematopoiesis, they may have limitations as human disease models, similar to GATA1low mice or TPO-overexpressing mice.13-15
Figure 1.
Frameshifting point mutation in MYB gene in granulocytic DNA from a patient with myelofibrosis with myeloid metaplasia. Insertion of a cytosine base (arrow; c.1992insC and p.S599EfsX605; GenBank reference sequences NM_005375 and NP_005366, respectively) leads to a frameshift with premature stop codon generation. This was the only nonsynonymous point mutation detected in analysis of MYB gene (25 patients) and EP300 KIX domain. Bottom: wild-type; middle: patient sense sequence; top: patient antisense sequence. Patient sequences demonstrate confused sequence expected for mixed clonality insertional mutation.
Supported by National Cancer Institute award K12 CA90628 to D.P.S. We thank Terra Lasho and Heather Powell for DNA extraction and cell bank curation, and Scott H. Kaufmann and Ayalew Tefferi for helpful advice.
References
- 1.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434: 1144-1148. [DOI] [PubMed] [Google Scholar]
- 2.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365: 1054-1061. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of Jak2 in myeloproliferative disorders. N Engl J Med. 2005;352: 1779-1790. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and agnogenic myeloid metaplasia. Cancer Cell. 2005;7: 387-397. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Gilliland DG. The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc. 2005;80: 947-958. [DOI] [PubMed] [Google Scholar]
- 6.Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106: 2162-2168. [DOI] [PubMed] [Google Scholar]
- 7.Steensma DP, Dewald GW, Lasho TL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106: 1207-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpinelli MR, Hilton DJ, Metcalf D, et al. Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc Natl Acad Sci U S A. 2004;101: 6553-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf D, Carpinelli MR, Hyland C, et al. Anomalous megakaryocytopoiesis in mice with mutations in the c-Myb gene. Blood. 2005;105: 3480-3487. [DOI] [PubMed] [Google Scholar]
- 10.Kasper LH, Boussouar F, Ney PA, et al. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature. 2002;419: 738-743. [DOI] [PubMed] [Google Scholar]
- 11.Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65: 677-689. [DOI] [PubMed] [Google Scholar]
- 12.Frampton J, McNagny K, Sieweke M, Philip A, Smith G, Graf T. v-Myb DNA binding is required to block thrombocytic differentiation of Myb-Ets-transformed multipotent haematopoietic progenitors. EMBO J. 1995;14: 2866-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan XQ, Lacey D, Hill D, et al. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88: 402-409. [PubMed] [Google Scholar]
- 14.Vannucchi AM, Bianchi L, Paoletti F, Di Giacomo V, Migliaccio G, Migliaccio AR. Impaired GATA-1 expression and myelofibrosis in an animal model. Pathol Biol (Paris). 2004;52: 275-279. [DOI] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood. 2002; 100: 1123-1132. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Boone TC, Murdock DC, et al. Studies of the human c-myb gene and its product in human acute leukemias. Science. 1986;233: 347-351. [DOI] [PubMed] [Google Scholar]