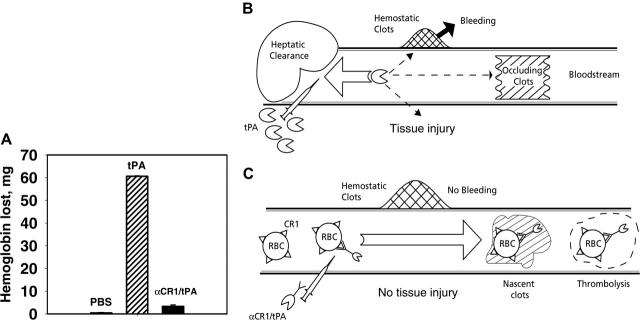
Figure 7.
Coupling of tPA to circulating RBCs reduces rebleeding. (A) Soluble tPA caused greater lysis of hemostatic clots than anti-CR1/tPA. Segments from the tails of TgN-hCR1 mice were amputated, and 5 minutes after hemostasis was attained, the tails were immersed into warm saline. PBS, tPA, or anti-CR1/tPA (2 mg/kg tPA each) was injected through the jugular vein. The amount of hemoglobin released from the tail over the ensuing hour was measured. (B, C) Schematic comparison of vascular delivery of tPA and anti-CR1/tPA. (B) Rapid clearance by liver, among other reasons, prohibits prophylactic use and dictates injection of large doses of tPA, which diffuses into hemostatic clots and tissues, causing bleeding and side effects. (C) Injected anti-CR1/tPA binds predominantly to RBCs, circulates for a prolonged time without access to preexisting hemostatic clots and extravascular tissues, while incorporating into and dissolving nascent intravascular clots.