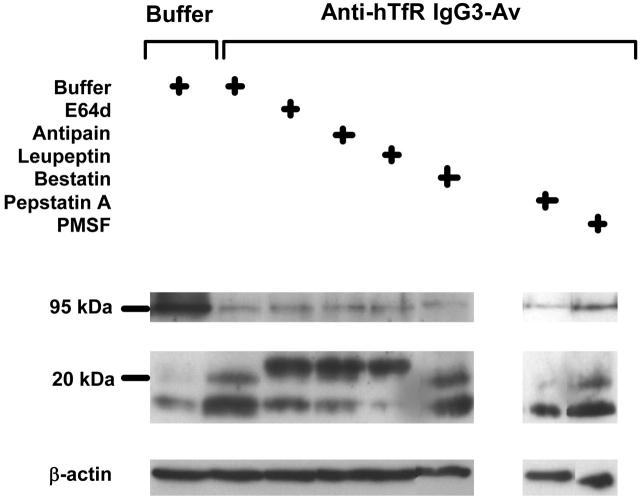
Figure 7.
A papainlike cysteine protease is involved in the degradation of TfR induced by anti-hTfR IgG3-Av. ARH-77 cells preincubated with buffer, 250 μM E64d, 250 μM antipain, 250 μM leupeptin, 250 μM bestatin, 500 μM pepstatin A, or 500 μM PMSF for 1 hour were treated with buffer or 50 nM anti-hTfR IgG3-Av for an additional 4 hours before being harvested. Cell lysates were analyzed by immunoblotting with a murine mAb against the intracellular N-terminus of the human TfR followed by incubation with goat anti-mouse IgG-HRP. The blot was then stripped and reblotted with a murine anti-β-actin mAb. The regions of the 95-kDa intact TfR, 16- to 23-kDa TfR fragments, and β-actin are shown. Except for the PMSF data, these experiments were repeated at least once with similar results.