Abstract
The diagnosis of anemia is an important aspect of the practice of hematology. The first step is to decide whether the patient is, in fact, anemic. Unless earlier blood counts are available, and they often are not, the physician must make his or her decision on the basis of the population distribution of hemoglobin values. How likely is it that the patient's hemoglobin value lies below the normal distribution; that is, “the lower limit”?
The definition of anemia has attracted considerable interest recently because of epidemiologic studies that suggest that anemia may be associated with poorer outcomes in a variety of disorders. In many studies the definition of anemia used is that suggested by a WHO expert committee nearly 40 years ago.1 Bearing the imprimatur of the WHO apparently carries much weight, although, as we shall point out, the numbers so casually presented in that document were based on very few data using methods that were inadequate. A reproduction of the entire section dealing with the establishment of this standard is shown in Figure 1. Cook et al2(p3362) recently lamented “the near universal acceptance of the WHO criteria of anemia.” As we will show, the acceptance of these standards is, indeed, to be decried. Table 1 summarizes the “lower limit of normal” offered by the WHO committee and various other sources.
Figure 1.

A reproduction of the page of the WHO report that has been used as a standard for anemia in epidemiologic studies. The casual treatment of the lower level of normal, given in whole numbers without decimal points in this 40-page document dealing with nutritional anemia, clearly suggests that the authors never intended for these values to become a hallowed international standard. (From Nutritional Anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405:1-401; with permission.)
Table 1.
Lower limits of normal of hemoglobin concentration of the blood of adult men and women as assessed by various sources
| Source | Men, g/dL | Women, g/dL | Percent normal below cutoff | Effect of race |
|---|---|---|---|---|
| WHO (Blanc et al1) | 13 | 12 | Not provided | Not provided |
| Jandl3 | 14.2 | 12.2 | 2.5 | Discussed |
| Williams (Beutler et al4) | 14.0 | 12.3 | 2.5 | Not provided |
| Wintrobe (Lee et al5) | 13.2 | 11.6 | Not provided | Not provided |
| Rapaport6 | 14 | 12 | Not provided | Not provided |
| Goyette7 | 13.2 | 11.7 | 5 | Blacks' hemoglobin 0.5 g/dL lower |
| Tietz8 | 13.2 | 11.7 | Not provided | Not provided |
| Hoffman et al9 | 13.5 | 12.0 | 2.5 | Not provided |
To convert hemoglobin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
The differences in the stated lower limits of normal may seem small, but in their application they are far from trivial. For example, assuming a Gaussian distribution of the data one may calculate that if the 142-g/L(14.2-g/dL) cutoff proposed by Jandl3 is correct, a man with a 130-g/L(13-g/dL) hemoglobin concentration, therefore passing the WHO standard for anemia, would be in the lowest 0.01% of the distribution of hemoglobin values. Conversely, if the WHO standards were correct, the cutoff value given by Jandl3 would result in adjudging 22% of all healthy men to be anemic.
How is one, then, to find a hemoglobin concentration above which 95% or 97.5% of the normal subjects are included? In the past, a part of the problem has been the availability of large databases that are sufficiently well documented to allow exclusion of individuals who are not “normal.” The systematic exclusion of such population members is important in dealing with an abnormality that is as common as is anemia, particularly in women. Fortunately, at least 2 such databases now exist. One is the NHANES-III (the third US National Health and Nutrition Examination Survey) database, the other the Scripps-Kaiser database that we collected in the San Diego area between 1998 and 2002.10,11 The ethnic origin of the individuals designated as white in these 2 databases is probably very similar. White Americans are a genetic mixture of persons from various parts of Europe and the Middle East. Data regarding ancestral origin are available in the Scripps Kaiser database but not in NHANES-III, so that direct comparison is not possible. Among black Americans there are varying degrees of admixture with non-African ancestry. The NHANES database attempts to reach a nationwide balance. It is likely that more of the black population of San Diego originates from the Northeast and the Midwest; therefore, San Diego may have greater white admixture than the NHANES sample. However, this has not been documented by the study of genetic markers in either data set. The NHANES-III database has recently been “mined” for hematologic values. The data in these compilations are valuable, but unfortunately the hemoglobin values in one report are derived from the entire population, not corrected for subjects with disease states that may be associated with anemia.12 The other report uses exclusion criteria that are so draconian that less than 40% of the subjects remain in the data set.13 Moreover, data derived from white subjects are not provided.
In these and many other previous analyses of hematologic data the subjects have been divided into age in deciles. This type of analysis is shown in Table 2. Because there are virtually no differences in hemoglobin values of men in the age range of 20 to 59 or in women aged 20 through 49 in our data or in the analysis of the NHANES-III hemoglobin values,12 we have used these age ranges representing younger adults, and the ages beyond these ranges to represent older adults. In any analysis, elimination of subjects from consideration is constrained by the data that are available. Both the Scripps-Kaiser and the NHANES databases included transferrin saturation and serum ferritin levels, and we were therefore able to exclude subjects with a transferrin saturation of less than 16% or a serum ferritin level less than 10 μg/L. This serves to eliminate most subjects with iron deficiency anemia and also many of those with the anemia of chronic inflammation, in which the transferrin saturation is also usually low. We were also able to determine whether excessive alcohol intake, diabetes mellitus, renal failure, or inflammatory markers (C-reactive protein in NHANES, erythrocyte sedimentation rate in Scripps-Kaiser, elevated serum ferritin, or leukocytosis) affected the hemoglobin concentrations in these populations. None of these had an effect in the younger subjects, but in older subjects a serum creatinine of more than 106 μM (1.4 mg/dL) and elevated inflammatory markers (ESR > 30 in women and ESR > 20 in men or CRP > 1 mg/dL) were associated with significantly lower mean hemoglobin values.
Table 2.
Mean hemoglobin (g/dL) by sex and age in white and black subjects
|
Scripps-Kaiser
|
NHANES III
|
|||||
|---|---|---|---|---|---|---|
| No. | Mean Hgb | SD | No. | Mean Hgb | SD | |
| White men, y | ||||||
| 20-29 | 250 | 15.43 | 0.77 | 332 | 15.51 | 0.96 |
| 30-39 | 894 | 15.24 | 0.85 | 389 | 15.29 | 0.95 |
| 40-49 | 2219 | 15.20 | 0.89 | 379 | 15.20 | 0.99 |
| 50-59 | 3346 | 15.10 | 0.93 | 356 | 15.17 | 1.01 |
| 60-69 | 3073 | 15.02 | 0.95 | 364 | 14.94 | 1.04 |
| 70-79 | 1976 | 14.75 | 1.07 | 315 | 14.70 | 1.15 |
| 80+ | 466 | 14.36 | 1.20 | 255 | 14.29 | 1.31 |
| White women, y | ||||||
| 20-29 | 240 | 13.41 | 0.80 | 322 | 13.57 | 0.88 |
| 30-39 | 809 | 13.59 | 0.86 | 402 | 13.66 | 0.86 |
| 40-49 | 1917 | 13.58 | 0.86 | 321 | 13.58 | 0.94 |
| 50-59 | 2829 | 13.62 | 0.86 | 312 | 13.76 | 0.94 |
| 60-69 | 3006 | 13.63 | 0.88 | 320 | 13.66 | 1.04 |
| 70-79 | 2051 | 13.66 | 0.94 | 421 | 13.68 | 0.97 |
| 80+ | 427 | 13.54 | 0.95 | 342 | 13.44 | 1.21 |
| Black men, y | ||||||
| 20-29 | 27 | 14.83 | 0.76 | 403 | 14.84 | 0.96 |
| 30-39 | 87 | 14.76 | 1.02 | 394 | 14.61 | 1.18 |
| 40-49 | 163 | 14.58 | 1.06 | 290 | 14.50 | 1.07 |
| 50-59 | 157 | 14.46 | 1.08 | 166 | 14.25 | 1.27 |
| 60-69 | 103 | 14.27 | 0.98 | 137 | 14.19 | 1.02 |
| 70+ | 32 | 14.44 | 1.06 | 98 | 13.75 | 1.15 |
| Black women, y | ||||||
| 20-29 | 17 | 13.26 | 0.75 | 321 | 12.78 | 0.92 |
| 30-39 | 68 | 12.97 | 0.93 | 339 | 12.77 | 1.11 |
| 40-49 | 120 | 12.88 | 0.91 | 244 | 12.87 | 1.05 |
| 50-59 | 135 | 12.96 | 0.89 | 146 | 13.07 | 0.95 |
| 60-69 | 90 | 13.03 | 0.91 | 161 | 12.89 | 1.01 |
| 70+ | 30 | 12.93 | 0.88 | 135 | 12.65 | 1.11 |
To convert hemoglobin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
Older subjects with elevated creatinine and ESR or CRP were thus excluded from our calculations. Pregnant women were also excluded. Overall, the percentage of subjects excluded from the analysis ranged from 10% to 30%, except for black subjects in NHANES, in which the exclusion rate was approximately 50% for older men and 40% for women.
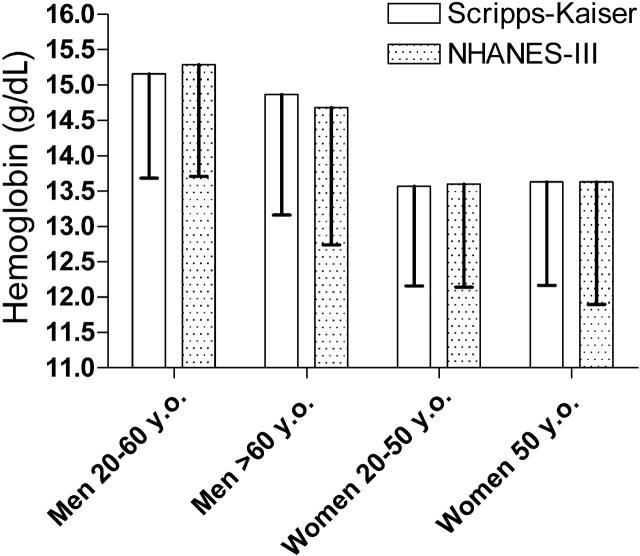
The mean and 1.65 standard deviation below the mean of the hemoglobin values of the white subjects are shown in Figure 2; this provides the hemoglobin concentration below which only 5% of normal subjects in the population will be found, given the Gaussian distribution of hemoglobin values. Table 3 presents the lower limit of normal for white and black men and women. These data are presented to indicate the 2.5th and the 5th percentile as the actual percentile in the data set and as the predicted value based on the assumption that the hemoglobin values have a Gaussian distribution, as assumed in Figure 2. This assumption is clearly valid, as shown by the excellent correspondence between the predicted cutoff and the actual cutoff based on the number of cases found.
Figure 2.
The mean and -1.65 standard deviations of the hemoglobin values of the white subjects in the Scripps-Kaiser and NHANES-III databases after applying the criteria described in the text. The bottom of the error bars represents the hemoglobin value below which only 5% of normal values would be predicted to be present.
Table 3.
Lower limits of normal for hemoglobin concentration of the blood in g/dL of younger (age 20-59 for men; 20-49 for women) and older white and black adults
|
Scripps-Kaiser
|
NHANES-III
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | 2.5% actual | 2.5% normal distribution | 5% actual | 5% normal distribution | No. | 2.5% actual | 2.5% normal distribution | 5% actual | 5% normal distribution | |
| White men, y | ||||||||||
| 20-59 | 6709 | 13.4 | 13.4 | 13.7 | 13.7 | 1456 | 13.4 | 13.4 | 13.8 | 13.7 |
| 60+ | 5515 | 12.8 | 12.8 | 13.2 | 13.2 | 934 | 12.2 | 12.4 | 12.8 | 12.7 |
| White women, y | ||||||||||
| 20-49 | 2966 | 11.9 | 11.9 | 12.2 | 12.2 | 1045 | 12.0 | 11.9 | 12.2 | 12.1 |
| 50+ | 8313 | 11.9 | 11.9 | 12.2 | 12.2 | 1395 | 11.5 | 11.6 | 12.0 | 11.9 |
| Black men, y | ||||||||||
| 20-59 | 434 | 12.6 | 12.5 | 12.9 | 12.9 | 1253 | 12.3 | 12.4 | 12.8 | 12.8 |
| 60+ | 135 | — | 12.4 | — | 12.7 | 235 | 11.4 | 11.9 | 11.8 | 12.2 |
| Black women, y | ||||||||||
| 20-49 | 205 | 11.2 | 11.2 | 11.5 | 11.5 | 904 | 10.9 | 10.8 | 11.3 | 11.1 |
| 50+ | 255 | 11.2 | 11.2 | 11.5 | 11.5 | 442 | 11.0 | 10.9 | 11.3 | 11.2 |
To convert hemoglobin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
— indicates insufficient numbers to determine.
The results obtained from the 2 databases are in very good agreement, but there are some minor differences that reach statistical significance. In particular, the mean hemoglobin of older men is slightly higher in the Scripps-Kaiser data than in the NHANES data. The latter difference in Table 3 is clearly an artifact resulting from the consolidation of all subjects older than 60 years into one group. As shown in Table 2, there are a higher proportion of men older than 80 years in the NHANES survey than in the Scripps-Kaiser data, and there is a slight but well-documented decrease in the mean hemoglobin concentration of each decile among men.12 Some minor discrepancies could also be due to differences in ethnic origin within populations studied. This would obviously be true with the population designated “black,” because it is well known that the genes of individuals classified as African American could be anywhere between 10% and 90% of European origin, with most of the remaining genome being of African origin. As pointed out earlier, differences in the degree of the European admixture could well account for the small differences in hemoglobin concentrations documented in the 2 data sets. This difference in genetic composition could also extend to the population designated “white”: we have previously shown that there is a difference of about 2.8 g/L (0.28 g/dL) in the average hemoglobin concentration of a white subject with northern versus southern European ancestry.14 This differential between northern and southern Europeans is maintained when the exclusion criteria used here are applied (data not shown).
How is it that the WHO standard, so widely used in epidemiologic studies, is so seriously flawed? In fairness to the Expert Committee, a group of distinguished hematologists and nutritionists, it is clear that it was not their intent to set up a hallowed standard for all to follow. The values defining anemia are given in a small table (Figure 1) and are presented without decimal points, clearly as approximations to be used within the context of the nutritional studies that were the principal topic of the meeting. The few references that are given to document this choice of numbers are, in general, surveys of the relatively small number of subjects, without documentation of methodology, and without efforts being made to remove patients with anemia in calculating the values. To further put these studies into the context of the times,15 the dilution of blood, sometimes obtained by finger puncture,16 was carried out with pipettes that were often inaccurate. Cyanmethemoglobin standards were not widely available, and calibration required oxygenation of a blood sample to serve as a standard with measurement of the amount of oxygen released by acid in a van Slyke apparatus. Needless to say, this procedure was not always carried out, and, as a consequence, results were often unreliable. In view of these difficulties, perhaps we should marvel not only at the fact that the WHO standard is still used but also that it was not further from the mark.
What, then, are reasonable benchmarks for anemia for a clinician to use today, based on today's improved laboratory practice and the data that we have been able to marshal from the Scripps-Kaiser and the NHANES study? The suggested lower limits of normal are summarized in Table 4. Based mostly on the larger Scripps-Kaiser database, but confirmed by the NHANES data, it would seem that a hemoglobin concentration below 137 g/L (13.7 g/dL) in a white man aged between 20 and 60 years would have only an approximately 5% chance of being a normal value. For older men, this hemoglobin value would be 132 g/L (13.2 g/dL). The corresponding value for women of all ages would be 122 g/L (12.2 g/dL). As shown in Table 1 and in a number of previous studies,17,18 the lower limit of normal of hemoglobin concentrations of African Americans are appreciably lower. Although some of this difference is a result of the high frequency of α-thalassemia in this population, other, as-yet-unidentified genes are involved, and because the gene frequency for α-thalassemia is extraordinarily high in the black population, and the diagnosis is not readily made, we judge it best not to censor such cases in determining the normal range for the black population.
Table 4.
Proposed lower limits of normal for hemoglobin concentration of the blood for white and black adults
| Group | Hemoglobin, g/dL |
|---|---|
| White men, y | |
| 20-59 | 13.7 |
| 60+ | 13.2 |
| White women, y | |
| 20-49 | 12.2 |
| 50+ | 12.2 |
| Black men, y | |
| 20-59 | 12.9 |
| 60+ | 12.7 |
| Black women, y | |
| 20-49 | 11.5 |
| 50+ | 11.5 |
Based on Scripps-Kaiser data for the 5th percentiles given in Table 2. NHANES data are considered to be confirmatory.
To convert hemoglobin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
Acknowledgments
This is manuscript number 17607-MEM from the Scripps Research Institute.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-07-3046.
Supported by the National Institutes of Health (grant DK53505-08) and the Stein Endowment Fund.
References
- 1.Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405: 1-40. [PubMed] [Google Scholar]
- 2.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101: 3359-3364. [DOI] [PubMed] [Google Scholar]
- 3.Jandl JH. Blood. Boston, MA: Little, Brown and Company; 1996.
- 4.Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U. Williams Hematology. New York, NY: McGraw-Hill; 2001.
- 5.Lee GR, Foerster J, Lukens J, Paraskevas F, Greer JP, Rodgers GM. Wintrobe's Clinical Hematology. Baltimore, MD: Williams and Wilkins; 1998.
- 6.Rapaport SI. Introduction to Hematology. Philadelphia, PA: JB Lippincott Company; 1987.
- 7.Goyette RE. Hematology. A Comprehensive Guide to the Diagnosis and Treatment of Blood Disorders. Los Angeles, CA: Practice Management Information Corporation (PMIC); 1997.
- 8.Tietz NW. Clinical Guide to Laboratory Tests. Philadelphia, PA: WB Saunders Co; 1995.
- 9.Hoffman R, Benz EJ Jr, Shattil SJ, Furie B. Hematology: Basic Principles and Practice. New York, NY: Churchill-Livingstone; 2004.
- 10.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of the 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359: 211-218. [DOI] [PubMed] [Google Scholar]
- 11.Waalen J, Felitti VJ, Gelbart T, Ho NJ, Beutler E. Penetrance of hemochromatosis. Blood Cells Mol Dis. 2002;29: 418-432. [DOI] [PubMed] [Google Scholar]
- 12.Hollowell JG, Van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Hematological and iron-related analytes: reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat. 2005;11: 1-156. [PubMed] [Google Scholar]
- 13.Cheng CK, Chan J, Cembrowski GS, Van Assendelft OW. Complete blood count reference interval diagrams derived from NHANES III: stratification by age, sex, and race. Lab Hematol. 2004; 10: 42-53. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, Felitti V, Gelbart T, Waalen J. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br J Haematol. 2003;120: 887-893. [DOI] [PubMed] [Google Scholar]
- 15.Beutler E. Hemoglobin level and red blood cell count findings in normal women. JAMA. 1958; 167: 1551. [DOI] [PubMed] [Google Scholar]
- 16.Natvig K. Studies on hemoglobin values in Norway, V: hemoglobin concentration and hematocrit in men aged 15-21 years. Acta Med Scand. 1966; 180: 613-620. [PubMed] [Google Scholar]
- 17.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and α-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106: 740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry GS, Byers T, Yip R, Margen S. Iron nutrition does not account for the hemoglobin differences between blacks and whites. J Nutr. 1992;122: 1417-1424. [DOI] [PubMed] [Google Scholar]