Abstract
Proteorhodopsin is the membrane protein used by marine bacterioplankton as a light-driven proton pump. Here, we describe a rapid cooperative assembly process directed by universal electrostatic interactions that spontaneously organizes proteorhodopsin molecules into ordered arrays with well defined orientation and packing density. We demonstrate the charge density-matching mechanism that selectively controls the assembly process. The interactions among different components in the system are tuned by varying their charge densities to yield different organized transmembrane protein arrays: (i) a bacteriorhodopsin purple membrane-like structure where proteorhodopsin molecules are cooperatively arranged with charged lipids into a 2D hexagonal lattice; (ii) selected liquid-crystalline states in which crystalline lamellae made up of the coassembled proteorhodopsin and charged lipid molecules are coupled three-dimensionally with polarized proteorhodopsin orientation persisting through the macroscopic scale. Understanding this rapid electrostatically driven assembly process sheds light on organizing membrane proteins in general, which is a prerequisite for membrane protein structural and mechanistic studies as well as in vitro applications.
Keywords: cationic lipid–proteorhodopsin complexes, membrane protein arrays, membrane protein crystallization, light-driven proton pump, photovoltaics
Membrane proteins (MPs) play an essential role in determining the interactions of biological systems with their surroundings. As gatekeepers of cellular boundaries, they are involved with matter, energy, and information transport to maintain life activities, and also with drug delivery to cure diseases. MPs are estimated to represent 20–30% of the currently sequenced genomes (1), and are targets for ≈70% of all drugs in the market (2). Although the sequence and function of many MPs have become known, their structures have only been solved at a rate of ≈0.2% of that of soluble proteins (3). Little is known about how to harness their unique biological functions in practical devices.
Long-range ordering is the prerequisite for structure determination, and unidirectional packing is needed for many in vitro applications depending on MP orientation because the activities of most MPs have vectorial aspects (4). The key problem that hinders MP assembly is their amphiphilic character. Except for a very limited number of MPs that form ordered crystals naturally, most MPs require detergents to free them from their native membranes. Although detergent-solubilized MPs with the surrounding detergent micelle in place could be crystallized (5), the orientation of MPs in those crystals is more or less isotropic, and the effect of lacking the biological membrane confinement on MP structure and mechanistic properties is always a concern. Detergent-assisted reconstitution is the most commonly used strategy to form 2D proteolipsome arrays (6). The MP order in those arrays depends critically on the detergent removal kinetics. Moreover, the lipid versus protein ratio, pH, and presence of salt and other small solutes also play key roles. In general, a formidable array of parameters needs to be mapped out for different MPs. The reconstitution process can be easily trapped within the states of unoriented or amorphous proteolipsome/detergent assemblies (5–8). Three-dimensional MP assembly with orientation control is possible only when directionally assembled 2D MP arrays are stacked unidirectionally, which is notoriously difficult (5). In very few cases, slow detergent removal from weeks to months leads to the formation of 3D stacked MP lamellae, but opposite MP orientations exist (9, 10). An alternative method using a high concentration of neutral lipid, which is expected to give a quasisolid bicontinuous cubic phase in the absence of MP, was used to crystallize bacteriorhodopsin (bR) (11, 12). The incorporated bR molecules appeared to slowly diffuse within the cubic framework and stack relative to each other through polar interactions to form small isolated bR crystals. Unfortunately, this process takes weeks to achieve because of the slow diffusion rate of bR in the viscous media.
Recently, a new family of photoactive transmembrane proteins, known as proteorhodopsin (PR), have been identified in seawater (13, 14). PR is a light-driven proton pump used by marine planktons to transport protons across their cell membranes. Unlike the homologous bR found in purple membranes of archaeal Halobacterium salinarium (15), PR is readily engineered and expressed in Escherichia coli in large quantity. More importantly, PR can be stably extracted from expression hosts by detergents, whereas solubilized bR is known to have a short shelf life (16). The advantages of PR over bR address a number of concerns associated with bR commercialization. PR has already been demonstrated as a viable security ink (17). It may also be possible to use PR in substitution of bR for biomimetic ATP production (18), optical data storage (19), artificial retinas (20), and photovoltaics (21).
For applications relying on a photoinduced proton gradient, the coherent orientation of the proton pumps within membranes is necessary so that their pumping actions do not offset each other. Moreover, instead of 2D reconstitution of proton pumps into bilayers of lipid vesicles with limited and isolated internal volumes, 3D unidirectional assembly offers the sequential addition of proton gradients from individual proteogradient membranes. Because bR in purple membranes is well oriented natively, numerous reports focused on 3D stacking of purple membrane patches using approaches such as electric sedimentation (22), Langmuir–Blodgett transfer (23), electric field directed loading (24), sequential deposition with polyelectrolytes (25), etc. Unfortunately, the interdigitation of different patches along the stacking direction and the presence of randomly oriented patches are intrinsic problems.
We describe here a rapid cooperative assembly process directed by universal electrostatic interactions that organizes PR spontaneously into ordered condensed phases. Although tuning the electrostatic interactions by pH and ionic strength are generally adopted for protein crystallization, it has been recognized that those conditions depend entirely on the specific nature of proteins, are unpredictable and can only be painstakingly sought by trial and error experiments (26). We demonstrate in this paper the governing concepts that make it possible to rationally and selectively control the assembly process leading to integrated 2D or 3D long-range-ordered PR-lipid arrays. Like all transmembrane proteins, PR possesses a spatially defined hydrophobic domain that spans the membrane wall, and charged intracellular/extracellular hydrophilic loops. From this perspective, it resembles a triblock copolymer with hydrophilic end groups and a hydrophobic interior region. The cooperative assembly of MPs with lipids in general is defined by the collective interplay of three major interactions: MP–MP, MP–membrane and membrane–membrane interactions. The electrostatic interactions in the system, if tuned properly, might play a dominant role for 2D and 3D assembly of MPs and lipids within the constraint of the van der Waals interactions, which always favor the membrane-embedded states for MPs. In contrast to the kinetically limited detergent-assisted reconstitution or quasisolid lipid cubic phase crystallization based on nonspecific van der Waals and hydrophobic interactions, we hypothesize that a charge density matching mechanism can be used to control the rapid electrostatically driven assembly of MPs with charged lipids once MPs and lipid solutions are put in contact.
Because many MPs have an isoelectric point <7 and are overall anionically charged under normal reconstitution conditions, we use oppositely charged cationic lipids instead of anionic or neutral lipids as often reported (5–8). The charge density of PR hydrophilic extramembrane domains can be tuned, e.g., the two extramembrane domains can be oppositely charged or likely charged to different degrees depending on pH, the loop sequences, and the choice of electrolytes that selectively attach to them. The charge density matching directs the 2D and 3D assembly of different charged components by maximizing the entropy gain from counterion release. As we demonstrated previously in this context, the cooperative assembly of charged inorganic species with charged surfactants induced the formation of ordered mesostructures at surfactant concentrations much lower than that required to form ordered surfactant liquid crystal phases (27, 28). This mechanism has also been shown to organize uniformly charged, water-soluble biopolymers on the surfaces of oppositely charged lipid membranes into well ordered phases (29–32).
We show here that the charge density matching between PR and membranes can be used to drive the spontaneous assembly process that packs PR into lipid bilayers whereas “squeezing out” the detergent originally associated with its membrane-spanning domain. The electrostatically driven assembly process can occur within seconds to generate highly ordered PR-lipid assemblies. No kinetically limited detergent removal via external means such as dialysis or hydrophobic resin extraction is needed. We further show that the interplay of the abovementioned three major interactions is electrostatically tunable by varying the charge density of PR extramembrane domains to spontaneously yield 2D or 3D long-range-ordered PR arrays with well defined orientation and packing density (Fig. 1).
Fig. 1.
Summary of the directed cooperative assembly of PR with cationic lipids. (A) PR trimers solubilized by DDM are spontaneously assembled into cationic lipid membranes to form a multilamellar structure (C and N terminus of PR indicated as red and green). The DDM molecules originally associated with the PR hydrophobic domain are “squeezed out” (data not shown). Depending on the effective charges associated with PR extramembrane domains, the stacking layers are either closely bound or osmotically swollen. (B and C) PR trimers in lipid membranes are organized into either a 2D rectangular (B) or hexagonal (C) lattice. The 3D PR arrays are polarized throughout the complexes.
Results and Discussion
PR Expression and Characterization.
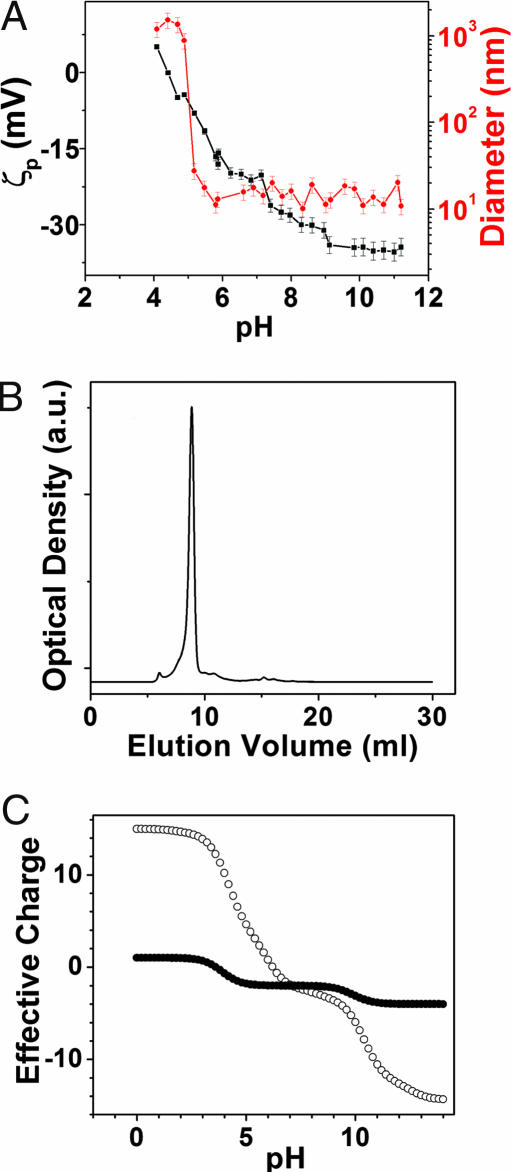
We expressed PR (BAC31A8) with a polyhistidine tail in the C terminus (33) in addition to its native sequence (13). In Escherichia coli cell membranes it functionally pumps protons as revealed by the acidification of the solution upon illumination (34). We studied the size and ζ-potential (ζp) of the n-dodecyl β-d-maltoside (DDM)-solubilized PR as a function of pH (Fig. 2A). From pH 5 to 11, an average diameter slightly >10 nm is observed (red trace). When the pH drops below 5, PR is rapidly precipitated to form micron size aggregates. The largest aggregation occurs around the isoelectric point, which is 4.5 as revealed by ζp titration curve (black trace). Gel filtration chromatography shows one dominant fraction at ≈300 kDa (Fig. 2B) independent of pH variations. The size of this fraction is ≈10 times that of PR molecular mass (≈29 kDa), indicating the DDM-solubilized PR is unlikely a monomeric form. We estimated the average number of DDM associated with each PR to be ≈150, which strongly suggests the DDM-solubilized PR is a trimer [see supporting information (SI)]. No optical spectrum changes were observed when DDM-solubilized PR solution was stored for more than a year. In contrast, surfactant-solubilized bR is a monomer (≈24 kDa) (35) and the solution shows progressive chromophore loss within a few days (16). The improved stability of solubilized PR over bR might be attributed to its trimeric form where the integrity of PR molecule is better maintained after release from membrane embedment.
Fig. 2.
Characterization of DDM-solubilized PR. (A) Average diameter (red trace) and ζ-potential (black trace) of solubilized PR measured as a function of pH. (B) Gel filtration of DDM-solubilized PR shows one dominant fraction with a calibrated molecular mass of ≈300 kDa. The size of this fraction was independent of pH. (C) Calculated pH-dependent effective charges associated with PR extramembrane domains based on its secondary structure model proposed by Beja et al. (13). The pI of extracellular side (●) and intracellular side (○) is 3.6 and 6.2, respectively.
Closely Stacked Cationic Lipid-PR (CL–PR) Multilamellar Structure with Polarized PR Orientation.
The expressed PR is asymmetric in terms of charge states of the two extramembrane domains. Calculations based on its secondary structure (13) show that the two extramembrane domains are oppositely charged in the pH range 3.8–6.2 (Fig. 2C). We first studied the cooperative assembly of PR and cationic membranes at pH 5. Rapid precipitation of reddish CL–PR complexes is spontaneous without centrifugation or detergent removal via external means (Fig. 3A Inset). The PR in the complexes has unchanged chromophore absorption as revealed by UV-Vis measurements. The complexes are found as birefringent particles of micron to submicron sizes when viewed under the cross-polarized optical microscope. No unprecipitated PR in supernatant is found when the number ratio of lipid molecules versus PR (defined as L/P) exceeds ≈20.
Fig. 3.
Assembly of 3D polarized PR arrays directed by the charge density-matching mechanism. (A) Synchrotron XRD of CL–PR complexes assembled at pH 5 (100% DOTAP, L/P = 60). Peaks under black arrows are harmonics of a closely bound stacking layer structure. Peaks under blue arrows are correlations from an in-layer PR 2D rectangular lattice. (Inset) pictures of DDM-solubilized PR before (left) and after (right) adding cationic lipids at pH 5. (B) TEM of CL–PR complexes (same as in A) stained with UA. (C) Synchrotron XRD of CL–PR complexes assembled at pH 7.0 (30/70 DOTAP/DOPC, L/P = 40). Peaks under black arrows are harmonics from a lamellar superlattice structure (q008 is too weak to be shown). Peaks under blue arrows are correlations from a PR 2D hexagonal lattice in each layer. Each peak is fitted and shown in dotted line.(Inset) blowout of the region from 0.17 to 0.37 Å−1. (D) Fourier reconstruction of the electron density distribution along the stacking-layer direction of the lamellar superlattice phase in C. Schematic representations of the membrane stacks are placed accordingly above the electron density distribution profile (headgroups of DOTAP and DOPC are shown as blue and white spheres, respectively).
Typical synchrotron x-ray diffraction (XRD) studies show that the complexes have a hierarchically ordered structure (Fig. 3A). Three equally spaced peaks (marked by black arrows) reveal a multilamellar structure with a periodicity of 54.1 Å (q001 = 0.116 Å−1). The coherent domain size (≈0.13 μm as estimated by Scherrer's equation) is comparable to the size of the complexes observed under the cross-polarized microscope. The membrane bilayer thickness (100% DOTAP) is ≈33 Å (29), which leaves ≈21.1 Å intermembrane space for the hydrophilic loops. The dimension of PR along the transmembrane direction can be estimated by homology with bR [≈55 Å (12)] and is about the same as the lamellar periodicity, indicating PR layers are closely stacked. Similar closely stacked CL–PR multilamellar complexes appear when PR spontaneously coassembles with cationic membranes of different compositions at this pH. Because the membrane–membrane like-charge repulsion always exists, we attribute this closely stacked state to the interlayer PR–PR interactions between oppositely charged PR extramembrane domains in adjacent layers through charge density matching, which maximizes the entropy gain by releasing the counterions originally associated with them.
PR molecules are directionally assembled within a given membrane layer to form oriented PR 2D lamellae (≈50 Å thick), presumably initiated by the interactions between their anionically charged extracellular domains and the cationic membranes; different lamellae are stacked relative to each other unidirectionally through PR “head-to-tail” charge interactions toward macroscopic scale (approximately micron) (Fig. 1A). Transmission electron microscope (TEM) studies using uranyl acetate (UA) as the staining agent also suggest the polarized PR orientation. UA is an anionic stain that preferably binds to the anionically charged PR extracellular domains. Typical TEM pictures show homogeneously spaced fringes with the periodicity of ≈56 Å (Fig. 3B), representing equally separated anionic extracellular domains with a spacing as the same as the stacking layer periodicity revealed by XRD (Fig. 3A). PR in each stacking layer form a well ordered 2D crystal that gives strong scatterings (peaks under blue arrows, Fig. 3A). The peaks are at 0.056, 0.082 and 0.173 Å−1, which fit nicely as q10, q01, and q12 of a 2D rectangular lattice (Fig. 1B).
The DDM molecules (DDMs) associated with PR trimers likely aggregate as toroid micelle surrounding their hydrophobic domains as discovered for DDM-solubilized H+-ATPase (36). The hydrophilic surfaces of DDM aggregates are energetically frustrated once PR trimers are coassembled into lipid membranes. We believe the DDMs are “squeezed out,” and possible inclusion of DDMs into cationic membranes is negligible because the strong PR-membrane and PR–PR interactions lead to the spontaneous precipitation of the closely stacked CL–PR complexes. This is true in the presence of DDM much more concentrated than its critical micelle concentration (CMC), in contrast to the generally accepted destabilization process for solubilizing MP at such detergent concentration (37).
CL–PR Lamellar Superlattice Structures with Controlled PR 3D Packing Density and Unidirectional PR Orientation.
The hypothesis that we could control the PR organization as desired by selectively modifying the charge densities was further confirmed as follows. When CL–PR complexes were assembled at a pH where both PR extramembrane domains are slightly negatively charged (pH 7; Fig. 2C), and a direct charge density matching between them is unavailable, we observed a completely transformed 3D structure. The synchrotron XRD of a typical CL–PR complex is shown, and the scattering peaks are fitted and individually plotted in dotted lines (Fig. 3C). Both the PR–PR and membrane–membrane electrostatic interactions become repulsive, pushing adjacent membrane layers apart. However, interlayer PR–membrane electrostatic interactions are attractive. Counterions are present to maintain charge neutrality in the system, which give rise to an osmotic pressure. The delicate balance between those forces leads to the formation of a new lamellar phase. Up to nine lamellar harmonics are observed (peaks under black arrows and the fitted peaks in black dotted lines, Fig. 3C). Compared with the XRD at pH 5 (Fig. 3A), two new harmonics appear before the expected first order Bragg peak (q003 = 0.114 Å−1; Fig. 3C). The first new harmonic (q001) is at 0.038 Å−1, suggesting that the periodicity of the new lamellar phase (d = 165.3 Å) is three times larger, reminiscent of a lamellar superlattice. Within each membrane, PR molecules are arranged into a highly ordered structure that gives a series of scatterings (peaks under blue arrows and the fitted peaks in blue dotted lines, Fig. 3C). The peaks are at 0.071, 0.123, 0.188, 0.213, 0.256, and 0.309 Å−1, corresponding to the q10, q11, q21, q30, q31, and q32 correlations of a 2D hexagonal lattice. Similar CL–PR lamellar superlattice structure appears when PR with both slightly anionically charged extramembrane domains is coassembled with cationic membranes of different compositions.
To better understand the structure of this new phase, we performed Fourier reconstruction of electron density ρ(z), where z is the direction perpendicular to the lamellar membranes (Fig. 3D). The phases and zero crossings of the form factor are determined as described (30, 32, 38). The electron densities of membrane wall (lipid tails, ≈0.30 e/Å3) and water (≈0.33 e/Å3) are known (30, 39). The transmembrane domain of PR has a higher electron density than lipid tails (≈0.30 e/Å3) because of the oxygen- and nitrogen-rich amino acids constituting the α-helices. The disordered extramembrane loops of PR are assumed to have a much lower electron density. For example, the electron density of flexible hydrocarbon is ≈0.16 e/Å3 (39). The membrane surface has the highest electron density [≈0.46 e/Å3 (30)], which is easily located by inspection of the ρ(z) map (schematic representation on top of Fig. 3D). The ρ(z) distribution across lipid bilayers displays an unusual feature: in contrast to a typical ρ(z) minimum (≈0.30 e/Å3) at midpoint (30, 32), an electron density plateau (≈0.32 e/Å3) is observed. The raised electron density is consistent with the presence of a transmembrane PR 2D lattice. Between adjacent membrane surfaces, there is a large spacing (≈117 Å) with an electron density maximum (≈0.34 e/Å3) in the middle. This number is slightly higher than the electron density of water (≈0.33 e/Å3), suggesting the presence of aqueous solution with counterions. The electron density decreases rapidly in the vicinity (≈20 Å) of membrane surfaces, indicating the highly flexible nature of PR extramembrane loops. We conclude that the CL–PR superlattice structure is an expanded lamellar phase consisting of coupled PR-membrane lamellae separated by aqueous solution.
The electrostatic tuning of intermembrane spacing offers an opportunity to adjust PR 3D packing density. For example, the charge density of PR extramembrane domains on C-termini can be further modified by selectively binding divalent cations to the polyhistidine tails. We studied the CL–PR complexes assembled in the presence of Ni2+ ions (typical molar ratio PR:Ni2+ = 1:100, and overall [Ni2+] ≈ 0.1 mM) under otherwise the same conditions. The interlayer PR–PR like-charge repulsion is reduced by binding cations, which changes the delicate balance among the previously discussed interactions and leads to the formation of a new lamellar superlattice (Fig. 4A). Six equally spaced harmonics appear (peaks under black arrows). The new lamellar periodicity is 118.5 Å (q001 = 0.053 Å−1), which is 46.8 Å smaller than the periodicity of the CL–PR complex assembled without Ni2+ at otherwise the same conditions (Fig. 3C). Interestingly, the 2D PR correlations within membranes are absent (a hump is barely seen between the first and second harmonic). This is likely caused by the flexibility of Ni2+-bound polyhistidine tails: the high-electron-density Ni2+ ions are randomly distributed, which average out the electron density correlations originally existing from the well positioned PR.
Fig. 4.
Modifying the charge density of PR extramembrane domains leads to additional polymorphic structures of CL–PR complexes. (A) Synchrotron XRD of the complexes with Ni2+-bound PR polyhistidine tags (30/70 DOTAP/DOPC, L/P = 40, PR:Ni2+ = 1:100, pH 7.0). The reduced PR–PR like-charge repulsion leads to the formation of a new lamellar superlattice phase (harmonics marked by black arrows). The PR in-layer correlation is barely seen (a weak hump marked by the blue arrow). (B) cryoTEM of CL–PR complexes with Ni2+-bound PR polyhistidine tag. (C) Synchrotron XRD of complexes assembled at pH 9 (30/70 DOTAP/DOPC, L/P = 40). No lamellar scattering is shown. Peaks under blue arrows are correlations of an in-membrane 2D hexagonally packed PR lattice analogous to that of bR in purple membranes. (D and E) Confocal microscopy shows fluorescently labeled PR and membrane in the purple membrane-like 2D PR crystals as in C, respectively. (Scale bars, 4 μm.)
The unidirectional orientation of PR in the lamellar superlattice phase persists as suggested by cryogenic TEM (cryoTEM) studies (Fig. 4B). Ni2+ ions bound on polyhistidine tails act as staining agents indicating the locations of PR C-termini. The cryoTEM of complexes without staining agents is generally featureless, but the Ni2+-bound complexes clearly show equally spaced fringes, representing equally separated PR C-termini along the stacking direction. The periodicity of the fringes is measured as ≈100 Å, close to what would be expected when Ni2+ ions are bound.
Purple Membrane-Like PR 2D Assembly.
To further test our charge density matching hypothesis, CL–PR complexes were assembled at even higher pH (pH 9), where the extramembrane domains are more negatively charged (Fig. 2C). At this charge density, a new PR assembly state appears (Fig. 4C). The interlayer PR–PR repulsion overrides the PR-membrane attractions, preventing lamellar stacking formation. Interestingly, the charge density matching between anionically charged PR extramembrane domains and the cationic membranes still drives the spontaneously assembly of PR molecules into cationic lipid bilayers, and highly ordered PR 2D crystals form (peaks marked by blue arrows). The scatterings are at 0.072, 0.125, 0.144, 0.190, 0.216, 0.259, 0.288, and 0.314 Å−1, corresponding to q10, q11, q20, q21, q30, q31, q40, and q32 correlations of a PR 2D hexagonal lattice, respectively. The strong PR correlations suggest coherent orientation analogous to bR in purple membrane patches (40). The diffuse peak centered at ≈0.14 Å−1 is likely the form factor from PR and lipid vesicles (Fig. 5). We studied the purple membrane-like PR assembly using confocal microscopy. FTIC and Liss Rhod PE are used to label PR (Fig. 4D) and membrane (Fig. 4E), respectively. The colocalization of PR and membrane is confirmed, and most PR 2D crystals are of micron sizes with irregular shapes. We expect those highly ordered PR 2D crystals are suitable for PR structural analysis using the approaches developed by Henderson et al. (41).
Fig. 5.
Synchrotron XRD of (A) DDM-solubilized PR, (B) 30/70 DOTAP/DOPC lipsome, and (C) PR and 30/70 DOTAP/DOPC mixture at pH 11 (L/P = 40). The diffuse scatterings are centered at ≈0.16, ≈0.12, and ≈0.14 Å−1, respectively. Neither ordered lamellar structure nor PR 2D organization shows up in C. The diffuse scattering in C is likely the combined form factor from PR and lipsomes.
Cationic Lipids and PR Mixtures Without Ordered Structure.
When the pH is raised further (e.g., pH 11), the DDM-solubilized PR is still stable, but both PR extramembrane domains become strongly negatively charged (Fig. 2C). As expected from our charge density-matching hypothesis, the charge density of lipid membranes no longer matches that of the PR extramembrane domains, and the intralayer PR–PR like-charge repulsion becomes dominant, which precludes even the formation of 2D ordered CL–PR arrays for membranes of all different charge density that we studied. No insoluble CL–PR complexes phase was observed. Synchrotron XRD shows neither ordered lamellar structure nor PR 2D correlations (Fig. 5C).
Conclusions
We have been able to create a rapid coassembly process directed by molecular electrostatic charge interactions to organize PR with charged lipids spontaneously. We have shown that the charge density matching mechanism can be used to selectively control the assembly process toward 2D and 3D long-range-ordered PR arrays. By tuning the intralayer charge density matching between PR and charged lipids, PR can be coassembled into cationic lipid membranes to form 2D ordered arrays without the need of detergent removal by external means; By tuning the interlayer charge density matching between PR extramembrane domains, the 2D PR lamellae can be coupled in 3D with well defined PR orientation and packing density. The interplay of three major interactions (PR–PR, PR–membrane and membrane–membrane interactions) is demonstrated to be electrostatically tunable and gives rise to the rich polymorphic phases of CL–PR complexes.
This rapid electrostatically driven assembly process sheds light on organizing MPs in general with charged lipids, where charged hydrophilic functionalities of MPs decorate charged membrane surfaces. We view the CL–PR assembly process as a paradigm in which charge heterogeneities are capable of inducing correlations and polarizations that are key for functional supramolecular assemblies (42). Stacked membrane structures mediated by MPs are abundant in biological systems. For example, the grana structure of thylakoid membranes in plant chloroplasts enables efficient light harvesting and development of a proton gradient for ATP production (43); myelin is used in higher-vertebrate nervous systems to pack nerve fibers (axons) where mutations in the extracellular domains of the integral MPs are the causes of many peripheral-nerve diseases (44). We believe that the governing concepts we have outlined here might also be the basis in the hierarchical assembly of membranes and associated MPs for sophisticated biological functions.
Materials and Methods
Cooperative Assembly of PR with Cationic Lipids.
PR was expressed and purified as reported (33). The PR gene sequence from the SAR86 group (13) was obtained synthetically and cloned with the pTrcHis2-TOPO TA Expression kit (Invitrogen, Carlsbad, CA). To obtain PR with a polyhistidine tail, the stop codon in the end of the native proteorhodopsin gene was mutated such that the expressed protein has a polyhistidine tail (KKGEFEAYVEQKLISEEDLNSAVDHHHHHH) in its C terminus coming from the myc epitope of pTrcHis2-TOPO plasmid. Cationic lipsome membranes consist of binary mixtures of the positively charged lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and the zwitterionic “helper” phospholipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) (Avanti Polar Lipids, Alabaster, AL) were prepared as described (45). The DDM-solubilized PR (5 mg/ml) and lipsomes (20 mg/ml) were mixed at controlled stoichiometric ratios and pH in >20-fold volume of buffer solution containing 0.05% DDM. The spontaneous assembly process accomplishes within seconds to minutes depending on the charge density of the assembly components. We observed similar CL–PR polymorphic structure variations when the charge density of PR hydrophilic domains was tuned for other PR variants, such as PR with no polyhistidine tail and PR with different charged amino acids mutations in their hydrophilic domains.
Gel Filtration Chromatography.
The gel filtration was done on BioCAD system with Superdex 200 10/300 GL column (GE Life Sciences, Piscataway, NJ) that has a size separation range from 10 to 600 kDa. The protein was eluted by PBS buffer with 0.05% DDM at different pH. Standard protein kit (Bio-Rad, Hercules, CA) containing bovine thyroglobulin, γ-globulin, chicken ovalbumin, horse myoglobin, and vitamin B12 were used to make the calibration curve.
ζ-Potential Measurements and Size Characterization by Dynamic Light Scattering.
Zetasizer (Nano-ZS; Malvern Instruments, Southborough, MA) was used to measure ζ-potential and size of DDM-solubilized PR using the titration mode. The concentration of PR was 0.5 mg/ml. PR was dissolved in 6 mM KNO3 solution with 0.05% DDM. The titration series started at pH ≈7, and went both ways (below and above the starting pH) respectively for separated samples. At each pH, the ζ-potential and size were measured three times and averaged.
XRD.
XRD measurements were as described before (45). The XRD data were fitted by the nonlinear least square method using Igor Pro (WaveMetrics, Portland, OR). The background was fitted by a power law function, and each scattering peak was fitted by the pseudoVoight function. The fitting was done by using the FuncFit routine in Igor Pro.
TEM.
The FEI Tecnai G2 Sphera Microscope was used. To stain CL–PR complexes, the complex suspension was added onto a holey carbon grid (Ted Pella, Redding, CA) and blotted from the other side. 5 μl 1% uranyl acetate was the added and blotted similarly after 1 min. For cryoTEM, the complex suspension was added onto the lacey formvar/carbon grid (Ted Pella), blotted, plunged into a cryogenic mixture (60/40 propane/ethane), and transferred to the 626 cryotransfer sample holder (Gatan, Pleasanton, CA).
Confocal Microscopy.
The Leica TCS SP confocal microscope (Leica Microsystems, Wetzlar, Germany) was used. Fluorescein-5-isothiocyanate (FITC; Invitrogen) was used as the PR dye following the protocol recommended by the supplier. 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Liss Rhod PE; Avanti Polar Lipids) was used as the membrane dye. The weight ratio of the fluorescent lipid versus total lipid content was set as 0.2%.
Supplementary Material
Acknowledgments
We acknowledge Prof. C. R. Safinya's group for help with synchrotron XRD experiments and R. van Zanten in Prof. J. A. Zasadzinski's group for help with cryoTEM experiments. This work was supported by a University of California Discovery Grant and Genencor International. Part of this work made use of facilities in the Materials Research Laboratory at University of California, Santa Barbara, which is supported by the Materials Research Science and Engineering Centers Program of the National Science Foundation under Award DMR05-20415. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
Abbreviations
- MP
membrane protein
- PR
proteorhodopsin
- bR
bacteriorhodopsin
- CL–PR complexes
cationic lipid-proteorhodopsin complexes
- XRD
x-ray diffraction
- TEM
transmission electron microscopy
- DDM
n-dodecyl β-D-maltoside
- CMC
critical micelle concentration.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702336104/DC1.
References
- 1.Wallin E, von Heijne G. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahlberg H, Fotiadis D, Scheuring S, Remigy H, Braun T, Mitsuoka K, Fujiyoshi Y, Engel A. FEBS Lett. 2001;504:166–172. doi: 10.1016/s0014-5793(01)02746-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU. Proc Natl Acad Sci USA. 2005;102:14278–14283. doi: 10.1073/pnas.0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eytan GD. Biochim Biophys Acta. 1982;694:185–202. doi: 10.1016/0304-4157(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 5.Ostermeier C, Michel H. Curr Opin Struct Biol. 1997;7:697–701. doi: 10.1016/s0959-440x(97)80080-2. [DOI] [PubMed] [Google Scholar]
- 6.Rigaud JL, Levy D. Methods Enzymol. 2003;372:65–86. doi: 10.1016/S0076-6879(03)72004-7. [DOI] [PubMed] [Google Scholar]
- 7.Jap BK, Zulauf M, Scheybani T, Hefti A, Baumeister W, Aebi U, Engel A. Ultramicroscopy. 1992;46:45–84. doi: 10.1016/0304-3991(92)90007-7. [DOI] [PubMed] [Google Scholar]
- 8.Hasler L, Heymann JB, Engel A, Kistler J, Walz T. J Struct Biol. 1998;121:162–171. doi: 10.1006/jsbi.1998.3960. [DOI] [PubMed] [Google Scholar]
- 9.Henderson R, Shotton D. J Mol Biol. 1980;139:99–109. doi: 10.1016/0022-2836(80)90298-3. [DOI] [PubMed] [Google Scholar]
- 10.Shi D, Hsiung HH, Pace RC, Stokes DL. Biophys J. 1995;68:1152–1162. doi: 10.1016/S0006-3495(95)80291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landau EM, Rosenbusch JP. Proc Natl Acad Sci USA. 1996;93:14532–14535. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch JP, Landau EM, Pebay-Peyroula E. Structure (London) 1999;7:909–917. doi: 10.1016/s0969-2126(99)80118-x. [DOI] [PubMed] [Google Scholar]
- 13.Beja O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich S, Gates CM, Feldman RA, Spudich JL, et al. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 14.Beja O, Spudich EN, Spudich JL, Leclerc M, DeLong EF. Nature. 2001;411:786–789. doi: 10.1038/35081051. [DOI] [PubMed] [Google Scholar]
- 15.Lozier RH, Bogomolni RA, Stoeckenius W. Biophys J. 1975;15:955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dencher NA, Heyn MP. Methods Enzymol. 1982;88:5–10. [Google Scholar]
- 17.Jensen RB, Kelemen BR, McAuliffe JC, Smith WC. 2004/0223323. US Patent. 2004:A1.
- 18.Luo TJM, Soong R, Lan E, Dunn B, Montemagno C. Nat Mater. 2005;4:220–224. doi: 10.1038/nmat1322. [DOI] [PubMed] [Google Scholar]
- 19.Brauchle C, Hampp N, Oesterhelt D. Adv Mater. 1991;3:420–428. [Google Scholar]
- 20.Chen ZP, Birge RR. Trends Biotechnol. 1993;11:292–300. doi: 10.1016/0167-7799(93)90017-4. [DOI] [PubMed] [Google Scholar]
- 21.Bertoncello P, Nicolini D, Paternolli C, Bavastrello V, Nicolini C. IEEE Trans Nanobiosci. 2003;2:124–132. doi: 10.1109/tnb.2003.813940. [DOI] [PubMed] [Google Scholar]
- 22.Kononenko AA, Lukashev EP, Chamorovsky SK, Maximychev AV, Timashev SF, Chekulaeva LN, Rubin AB, Paschenko VZ. Biochim Biophys Acta. 1987;892:56–67. [Google Scholar]
- 23.Miyasaka T, Koyama K, Itoh I. Science. 1992;255:342–344. doi: 10.1126/science.255.5042.342. [DOI] [PubMed] [Google Scholar]
- 24.Caplan SR, Fischer G. J Membr Sci. 1983;16:391–405. [Google Scholar]
- 25.He JA, Samuelson L, Li L, Kumar J, Tripathy SK. Langmuir. 1998;14:1674–1679. [Google Scholar]
- 26.McPherson A. Eur J Biochem. 1990;189:1–23. doi: 10.1111/j.1432-1033.1990.tb15454.x. [DOI] [PubMed] [Google Scholar]
- 27.Huo QS, Margolese DI, Ciesla U, Feng PY, Gier TE, Sieger P, Leon R, Petroff PM, Schuth F, Stucky GD. Nature. 1994;368:317–321. [Google Scholar]
- 28.Monnier A, Schuth F, Huo Q, Kumar D, Margolese D, Maxwell RS, Stucky GD, Krishnamurty M, Petroff P, Firouzi A, et al. Science. 1993;261:1299–1303. doi: 10.1126/science.261.5126.1299. [DOI] [PubMed] [Google Scholar]
- 29.Radler JO, Koltover I, Salditt T, Safinya CR. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 30.Wong GCL, Tang JX, Lin A, Li Y, Janmey PA, Safinya CR. Science. 2000;288:2035–2039. doi: 10.1126/science.288.5473.2035. [DOI] [PubMed] [Google Scholar]
- 31.Koltover I, Sahu S, Davis N. Angew Chem Int Ed. 2004;43:4034–4037. doi: 10.1002/anie.200460164. [DOI] [PubMed] [Google Scholar]
- 32.Yang LH, Liang HJ, Angelini TE, Butler J, Coridan R, Tang JX, Wong GCL. Nat Mater. 2004;3:615–619. doi: 10.1038/nmat1195. [DOI] [PubMed] [Google Scholar]
- 33.Kelemen BR, Du M, Jensen RB. Biochim Biophys Acta. 2003;1618:25–32. doi: 10.1016/j.bbamem.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Jensen RB, Kelemen BR. 2005/0095605A1. US Patent. 2005
- 35.Reynolds JA, Stoeckenius W. Proc Natl Acad Sci USA. 1977;74:2803–2804. doi: 10.1073/pnas.74.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auer M, Scarborough GA, Kuhlbrandt W. Nature. 1998;392:840–843. doi: 10.1038/33967. [DOI] [PubMed] [Google Scholar]
- 37.Le Maire M, Champeil P, Moller JV. Biochim Biophys Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 38.Blaurock AE. J Mol Biol. 1971;56:35–52. doi: 10.1016/0022-2836(71)90082-9. [DOI] [PubMed] [Google Scholar]
- 39.Harper PE, Gruner SM. Eur Phys J E. 2000;2:217–228. [Google Scholar]
- 40.Henderson R. J Mol Biol. 1975;93:123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- 41.Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, Downing KH. J Mol Biol. 1990;213:899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- 42.Stupp SI, LeBonheur V, Walker K, Li LS, Huggins KE, Keser M, Amstutz A. Science. 1997;276:384–389. doi: 10.1126/science.276.5311.384. [DOI] [PubMed] [Google Scholar]
- 43.Dekker JP, Boekema EJ. Biochim Biophys Acta. 2005;1706:12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Neuron. 1996;17:435–449. doi: 10.1016/s0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 45.Liang HJ, Angelini TE, Braun PV, Wong GCL. J Am Chem Soc. 2004;126:14157–14165. doi: 10.1021/ja046718m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.