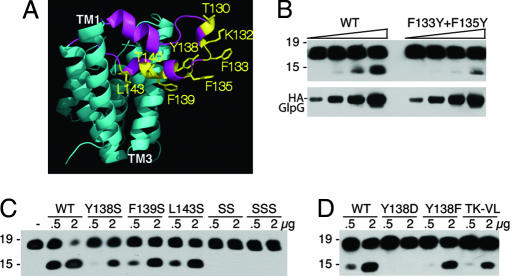
Fig. 2.
L1 loop residues that face toward membrane lipids are required for protease activity. (A) Lateral view of GlpG (2NRF) with L1 loop in magenta and its residue side chains that are expected to contact membrane lipid highlighted in yellow. (B) Effect of changing F133 and F135 to tyrosine on protease activity was compared with wild-type GlpG in a limiting enzyme dilution series (amounts used for each were 100 ng, 200 ng, 400 ng, and 800 ng). Anti-Flag Western blot analyses are shown, with mass standards in kilodaltons depicted to the left of each panel. GlpG levels in the reactions were compared directly by anti-HA Western blot analysis. (C) Y138, F139, and L143 were mutated to serine, and the effect on protease activity against C100Spitz-Flag was assessed. SS is a double Y138S+F139S mutant, whereas SSS carries all three residues mutated to serine. Two different amounts of enzyme (in micrograms) were assayed for each GlpG variant for 1 h, with the highest level of GlpG being approximately equimolar to substrate levels, and resulted in almost complete substrate cleavage by wild type (to assess whether mutants abolished activity). (D) Mutation of Y138 to aspartate or phenylalanine, as well as a double mutant T130V+K132L (TK-LV), reduced activity.