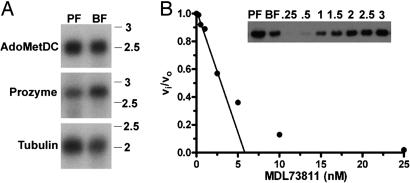
Fig. 2.
Analysis of AdoMetDC activity and expression in T. brucei lysates. (A) Northern blot analysis of mRNA (1 μg per lane) isolated from T. brucei cells for both AdoMetDC (Top) and prozyme (Middle) in procyclic form (PF), and blood form (BF) trypanosomes; tubulin (Bottom) is shown as a loading control. Molecular weight markers are given in kilobases. (B) Active-site titration of AdoMetDC in trypanosome BF cell lysates. Lysate (0.04 mg total protein; 0.01 ml at 4 mg/ml) was incubated with MDL 73811 (0–25 nM) for 1 h at 37°C. Remaining enzyme activity was determined (vi) and compared with the uninhibited activity (vo) in the lysate by quantitation of the amount of 14CO2 liberated from 1-14CO2-AdoMet. The concentration of AdoMetDC in lysates was estimated from the linear portion of the graph by the x-intercept (AdoMetDC = 6 nM). (Inset) Western blot of T. brucei AdoMetDC in PF and BF trypanosome cell lysates (0.04 mg of total protein per lane). Intensity of the 34-kDa band was compared with that measured for a titration of recombinant AdoMetDC (0.25–3 ng of protein) providing an estimate of the AdoMetDC protein concentration in the BF lysates (AdoMetDC = 5 nM). Based on the MDL 73811 titration and the Western blot analysis, the specific activity of AdoMetDC in the BF lysate can be estimated to be 0.8 s−1 (1.1 μmol/min/mg) at 0.04 mM AdoMet, which would correspond to ≈3 s−1 at saturating substrate (assuming the Km reported in Table 1).