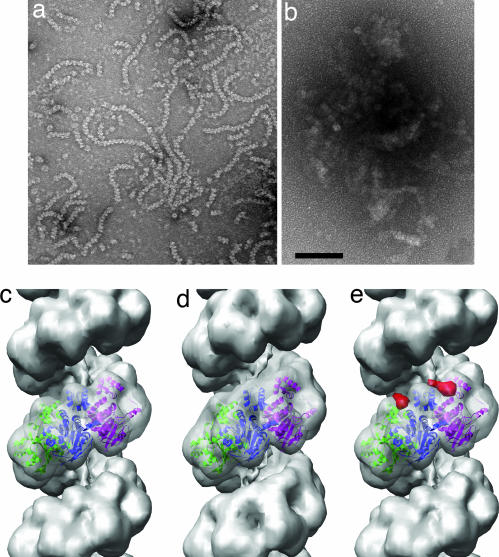
Fig. 1.
Electron microscopy of C. elegans RAD51–DNA filaments and their interaction with CeBRC-2. (a) In the presence of ATP, RAD51 forms filaments on single-stranded DNA that show the helical striations characteristic of all RecA-like filaments. (b) Incubation of RAD51–DNA filaments with full-length CeBRC-2 leads to massive aggregation of these filaments, with no free filaments found. The BRC 21–89 peptide induced a similar degree of aggregation (data not shown). (c–e) Three-dimensional reconstructions of pure RAD51–DNA filaments (c), and these filaments decorated with the BRC_60–89 peptide (d). A difference map (red density, e) between the BRC_60–89-RAD51–DNA filament (d) and the undecorated RAD51 filament (c) shows that BRC_60–89 is binding between the N-terminal domains of two adjacent RAD51 subunits. The difference map (e) is shown only for two subunits, as the slight difference in symmetry between the volumes in c and d requires that one look only at local differences. The atomic structure (28) of yeast RAD51 has been fit into the reconstructions in c–e, and each subunit is shown in a different color. (Scale bar: 1,000 Å.)