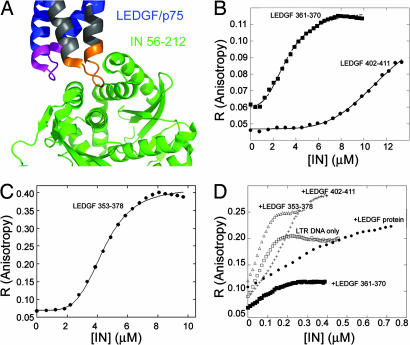
Fig. 1.
Ligand binding of IN: fluorescence anisotropy studies. (A) Crystal structure of the LEDGF–IN complex. The LEDGF-derived peptides used in this study are LEDGF 353–378 (gray), LEDGF 361–370 (orange), and LEDGF 402–411 (magenta). Coordinates are taken from ref. 18. (B and C) Fluorescence anisotropy-binding studies. IN was titrated into the fluorescein-labeled LEDGF/p75 peptides (100 nM): LEDGF/p75 361–370 and LEDGF/p75 402–411 (B) and LEDGF/p75 353–378 (C). Data were fit to the Hill equation. (D) The effect of the LEDGF/p75 and peptides derived from it on the DNA binding of IN. IN was titrated into fluorescein-labeled HIV-1 LTR DNA (10 nM) alone and in the presence of 1 μM LEDGF 361–370, LEDGF 353–378, LEDGF 402–411, and full-length LEDGF. Binding affinities and Hill coefficients are given in Table 2.