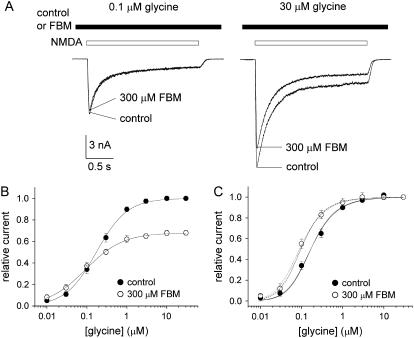
FIGURE 3.
FBM enhancement of glycine affinity to the NMDA channel. (A) NMDA currents were elicited by application of saturating concentrations of NMDA (1 mM) and different concentrations of glycine in the absence (control) and presence of 300 μM FBM. FBM clearly inhibits the current in the presence of 30 μM glycine but has only negligible inhibitory effect on the current with 0.1 μM glycine. (B) The relative current is defined as the ratio between the sustained current amplitude in different concentrations of glycine (and 0 or 300 μM FBM) and that in 30 μM glycine (and 0 FBM), and is plotted against the glycine concentration (n = 5–8). (C) The same data as in panel B, but the currents in 300 μM FBM are now normalized to the current in 30 μM glycine (and 300 μM FBM). The two very similar solid lines are the best fits to the mean data in the control condition using Eq. 2 with a p-value of 1 and an m-value of 5 or 3, respectively. The best fits give relatively constant Kgly values of 308 and 243 nM, respectively. With the same fixed parameters, the best fits (dotted lines) to the data in 300 μM FBM give Kgly = 165 (m = 5), and 129 nM (m = 3), respectively. We also fixed Kgly at 308 or 243 nM, and fit for the p-value in 300 μM FBM (dashed lines, p = 2.6). Note the close proximity between the dotted and dashed lines.