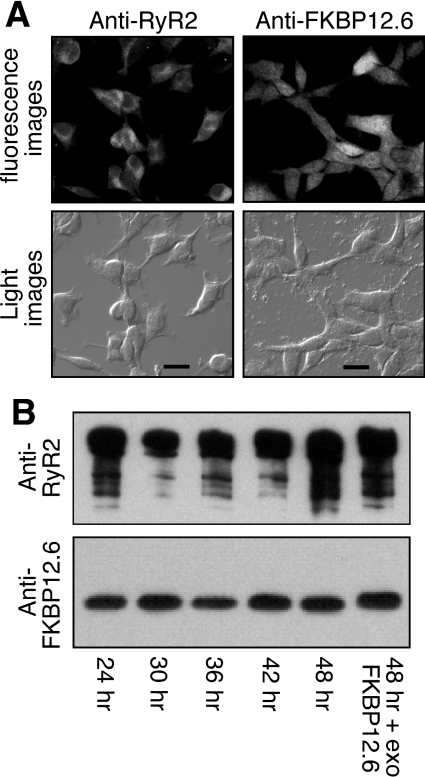
Figure 3. Characterization of HEK-293 cells co-expressing RyR2–FKBP12.6.
(A) HEK-293 cells expressing both RyR2 and FKBP12.6 were grown on glass coverslips. The cells were induced with tetracycline for 30 h and immunostained with either anti-RyR or anti-FKBP12/12.6 antibodies. Immunolabelling was visualized by fluorescent microscopy with the same exposure time for both protein signals (scale bar, 20 μm). (B) Western blot showing the interaction between RyR2 and FKBP12.6 at various induction times with tetracycline. The FKBP12.6–RyR2 complex was immunoprecipitated using the anti-RyR antibody followed by immunoblotting with the anti-RyR (upper panel) and anti-FKBP12/12.6 (lower panel) antibodies. Loadings to the SDS/PAGE gel were adjusted in an attempt to achieve similar loadings of RyR2 in each lane for easy comparison of the levels of associated FKBP12.6 between different induction times. The final lane included 200 nM exogenous FKBP12.6 in the immunoprecipitation to saturate the FKBP12.6-binding sites in RyR2. Results shown are representative of three separate experiments.