Scheme 1.
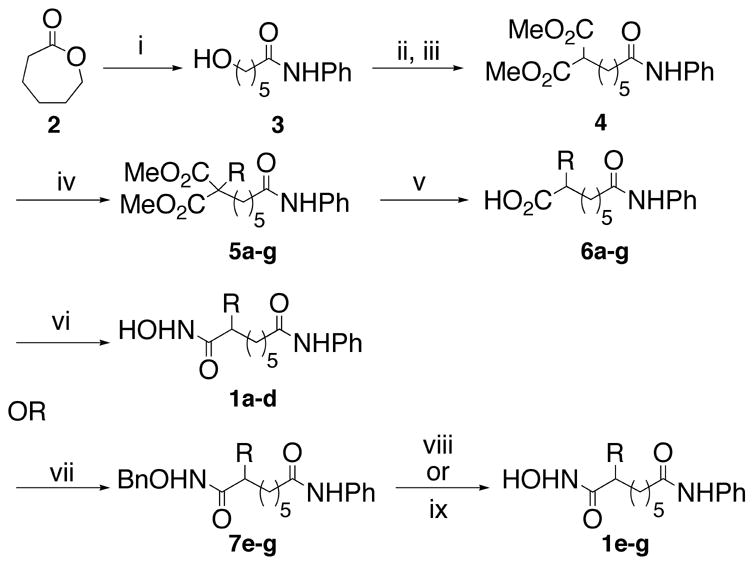
Reagents and conditions for the racemic synthesis of compounds 1a–g: (i) PhNH2, AlMe3, THF, 98%; (ii) MsCl, TEA, CH2Cl2, 99%; (iii) (a) NaH, Dimethylmalonate, THF; (b) mesylate from ii, THF, reflux, 90%; (iv) NaH, RX, THF, reflux, 33–98%; (v) (a) LiCl, H2O, DMSO, reflux; (b) NaOH, MeOH, reflux, 67–83%; (vi) Ethyl chloroformate, N-methylmorpholine, NH2OH, MeOH, 10–24%; (vii) CDI, TEA, NH2OBn, THF, reflux, 75–91%; (viii) H2, Pd/C, MeOH, 48%; (ix) BCl3, CH2Cl2, 84–85%.
