Abstract
We present a real-time PCR approach for the identification and subtyping of HLA-DR4 alleles. The technique, which uses sequence-specific primers and probes in conjunction with real-time PCR for the detection and differentiation of target alleles, is rapid, involves minimal hands-on time, and is inexpensive compared to existing methods. Further, there is no post-PCR handling, so the risk of contamination is avoided. We have validated the assay using 44 blinded and 56 unblinded samples, which were identified with 100% accuracy, sensitivity, and specificity. We demonstrate the applicability of this assay as an alternative approach to traditional HLA typing methods.
Keywords: HLA typing, Real-time PCR, HLA-DRB1*04, Type 1 Diabetes, Rheumatoid Arthritis, Genotyping, TaqMan
Introduction
The presence of specific Human Leukocyte Antigen (HLA)-DR4 alleles has been associated with susceptibility to several autoimmune diseases, including Type I diabetes (T1D) [Svejgaard et al., 1981; Nepom et al., 1983; Nepom et al., 1991; Noble et al., 1996], Rheumatoid Arthritis (RA) [Nepom et al., 1987; Wordsworth et al., 1989; Weyand et al., 1992; Wordsworth et al., 1992; Nepom et al., 1992], Addison’s Disease [Maclaran et al., 1986; Yu et al., 1999], and Pemphigus Vulgaris [Scharf et al., 1989]. While the frequency of DR4-positive patients is elevated in Caucasians and most other ethnic groups, population studies have shown that it is not the DR4 group, but rather specific DR4 alleles that confer susceptibility. The presence of these specific alleles is a useful marker in risk assessment. More importantly, it is a key element in the trimolecular complex (MHC-peptide-T cell receptor) and determines the repertoire of autoantigen epitopes that can be presented as well as the restricted subset of reactive T cells [Wordsworth et al., 1992; Doherty et al., 1998; Gebe et al., 2001; Nepom, 2001]. For these reasons, the identification of HLA haplotypes is important to many clinical studies of these autoimmune diseases. In our laboratory, the determination of HLA type is a critical first step for the selection of appropriate analytical reagents and assays for monitoring and phenotyping T cells. It is essential in many of our studies that typing be completed within a very short time-frame, preferably within a few hours of obtaining a fresh sample and must often be done using limited sample material. Since complete HLA typing by conventional methods is time-consuming and costly for screening large numbers of samples and only specific HLA subtypes are directly relevant in our studies, a rapid targeted approach to high resolution typing of DR4 is ideal.
Traditional HLA typing methods, such as serological typing, provide low level resolution of HLA type. Many molecular typing methods have been described, such as polymerase chain reaction (PCR) with sequence-specific oligonucleotide probes (PCR-SSO) [Saiki et al, 1986], which is also generally used for low level resolution. PCR with sequence-specific primers (PCR-SSP) [Olerup et al., 1992] is applicable for high resolution determinations but is labor intensive and time consuming. Likewise, sequence-based typing (PCR-SBT) is able to resolve HLA alleles with high resolution [Sayer et al., 2001] but requires significantly more time and handling than the method presented here. Further, all of the above molecular genotyping approaches require post-PCR handling which not only increases the required time but, more critically, introduces the potential for cross-contamination of samples and reagents. Since none of the existing methods were able to provide the information we needed in a rapid enough timeframe, we developed an approach using real-time PCR in conjunction with sequence-specific primers and probes for rapid HLA-DR4 subtyping. We suggest the name QPCR-SSPP for this technique—quantitative PCR with sequence-specific primers and probes.
Materials and Methods
Isolation of DNA
Peripheral blood or buccal swabs were obtained from healthy adults and autoimmune disease patients after obtaining informed consent. Genomic DNA was isolated using the QIAamp® DNA Blood Mini Kit (Qiagen, Inc.).
Primers and Probes
Primers and probes (shown in Table I) were designed within exon 2 of the HLA-DRB1 locus using Primer Express™ v2.0 (Applied Biosystems, Inc.), based on the sequence alignments of Release 2.14.0 (updated July 14, 2006) of the IMGT/HLA Sequence Database (see http://www.ebi.ac.uk/imgt/hla/align.html) [Robinson et al., 2003]. Pan-DR4 primers and probe were designed to selectively recognize all DR4 alleles for low level resolution. DR4 subtyping reactions were designed to use a single pair of primers with nine fluorescently-labeled probes in a total of 5 separate reactions. These nine probes identify the major DRB1 polymorphisms at codons 57, 71, 74, and 86 that, in combination, distinguish most DR4 alleles. The probes were labeled either with FAM™ (6-carboxyfluorescein) or VIC® and were modified with a minor groove binder (MGB) and a non-fluorescent quencher (Applied Biosystems, Inc.). The MGB group allows the use of shorter probes and enhances the sensitivity to mismatches. All reactions were carried out with DR4 group-specific primers which restrict amplification to DR4 alleles, the exceptions being DRB1*1122 and *1410; these rare alleles are predicted to be non-amplifiable by the pan-DR4 group-specific primers but should be amplified by the DR4 subtyping primer pair, giving a distinct pattern with the subtyping probe set which would allow them to be easily distinguished from DR4 alleles. The primers and probes were designated as GDR1 through GDR17 (Genotyping oligonucleotides for HLA-DR), as shown in Table I.
Table I.
Oligonucleotide sequences for high resolution HLA typing of DRB1*04 alleles.
| Oligo Name | Description | Sequence (5′ to 3′) | Final concentration (nM) |
|---|---|---|---|
| GDR1 | DRA forward primer | AGGCCGAGTTCTATCTGAATCCT | 225 |
| GDR2 | DRA reverse primer | CGCCAGACCGTCTCCTTCT | 225 |
| GDR3 | DRA probe | VIC-CATAAACTCGCCTGATTG-MGB | 50 |
| GDR4 | Pan DR4 forward primer | CGTTTCTTGGAGCAGGTTAAACA | 225 |
| GDR5 | Pan DR4 reverse primer | GCACGTACTCCTCTTGGTGATAGA | 225 |
| GDR6 | Pan DR4 probe | FAM-TCCGTCCCGTTGAAGA-MGB | 50 |
| DR4 subtyping: | |||
| GDR7 | DR4 subtyping forward primer | CGTTTCTTGGAGCAGGTTAAACA | 225 |
| GDR8 | DR4 subtyping reverse primer | CTCGCCGCTGCACTGTG | 225 |
| GDR9 | DRB codon 57-gat probe | FAM-CAGTACTCGGCATCAG-MGB | 50 |
| GDR10 | DRB codon 57-agc probe | VIC-ACTCGGCGCTAGG-MGB | 50 |
| GDR11 | DRB codon 71-aag probe | FAM-AGCAGAAGCGGGC-MGB | 50 |
| GDR12 | DRB codon 71-agg probe | VIC-TGGAGCAGAGGCG-MGB | 50 |
| GDR13 | DRB codon 71-gag probe | FAM-GAAGACGAGCGGGC-MGB | 50 |
| GDR14 | DRB codon 74-gcg probe | FAM-TGTCCACCGCGGC-MGB | 25 |
| GDR15 | DRB codon 74-gag probe | VIC-TGTCCACCTCGGC-MGB | 100 |
| GDR16 | DRB codon 86-ggt probe | FAM-AAGCTCTCACCAACC-MGB | 50 |
| GDR17 | DRB codon 86-gtg probe | VIC-AAGCTCTCCACAACC-MGB | 50 |
Real-time amplification
PCR amplification for DR4 screening (low-resolution typing) was carried out in a single well (using primers GDR1, 2, 4, and 5 and probes GDR3 and 6 from Table I), and DR4 subtyping was carried out in five additional wells, four of which were multiplex reactions that used pairs of allele-specific probes corresponding to the most common sequence variations at DRB1 exon 2 codons 57, 71, 74, and 86. Thus, each of these wells contained primers GDR7 and 8, and probes in pairs: GDR9 and 10, GDR11 and 12, GDR14 and 15, and GDR16 and 17, respectively. The fifth well contained a probe for an additional sequence variant at DRB1 codon 71 corresponding to the sequence present in DRB1*0402 (probe GDR13). The primers and probe for the DRA locus in well 1 (GDR1, 2, and 3) served as an internal control for amplification and template quality and quantity. Reactions were performed with approximately 15 ng of genomic DNA, allele or group-specific primers, and FAM and/or VIC-labeled probes in TaqMan® Universal Master Mix (Applied Biosystems, Inc.) in a final volume of 25 microliters per well. All primers and probes were used at the final concentrations listed in Table I. Reactions were amplified on an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Inc.) as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for15 sec, 60°C for 1 min.
An alternate set of primers was designed to carry out low resolution DR4 genotyping using the dye, SYBR®green I: DR4 primers, 5′-CGTTTCTTGGAGCAGGTTAAACA-3′ and 5′-GAAGCGCACGTACTCCTCTTG-3′ and DRA primers, 5′-AGGCCGAGTTCTATCTGAATCCT-3′ and 5′-AAATCTCATCACCATCAAAGTCAAAC-3′. These primers were used under the same amplification conditions as those described above for TaqMan assays, except that reactions were run in two wells—one for DRA and one for pan DR4—since SYBRgreen I reactions cannot be multiplexed. Primers were used at 300 nM, except for the DRA forward primer, which was used at 50 nM.
Analysis and interpretation
Thresholds were set (according to manufacturer’s guidelines) to measure fluorescence during the exponential phase of the PCR. Typically, thresholds were set at 0.05. Thresholds for SYBRgreen I reactions were set at 0.3. The cycle during which each sample reached this threshold level of fluorescence (CT) was determined. Reactions were scored as positive if the CT for DRA was no more than 34 and the CT for other targets was less than 40 and within 8–10 cycles of DRA. Generally, the CT for DRA was 24–26 and CTs for other targets were 25–35. A modified algorithm was used to interpret DR4 codon 74 results, since there was partial cross-reactivity between DRB1*0403 (and other alleles that share the polymorphic adenine at codon 74, such as DRB1*0407) and the GDR14 probe. Signal detected with GDR14 was scored as positive only if the CT was lower than the CT for GDR15. All algorithms were combined into a Microsoft® Office Excel (Microsoft Corp.) table for rapid analysis of subtyping results based on the amplification patterns detailed in Table II. We chose not to resolve DRB1*0403 from *0406 as neither is associated with susceptibility to T1D nor RA. However, all other common DR4 alleles are resolved individually, and most are also resolved in combination (i.e. DR4 heterozygotes).
Table II.
Interpretation of QPCR-SSPP results for common DR4 alleles.
| Probes
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRB1* | GDR3 | GDR6 | GDR9 | GDR10 | GDR11 | GDR12 | GDR13 | GDR14 | GDR15 | GDR16 | GDR17 |
| 0401 | + | + | + | + | + | + | |||||
| 0402 | + | + | + | + | + | + | |||||
| 040301 | + | + | + | + | + | + | |||||
| 040302 | + | + | + | + | + | ||||||
| 0404 | + | + | + | + | + | + | |||||
| 0405 | + | + | + | + | + | + | |||||
| 0406 | + | + | + | + | + | + | |||||
| 040701 | + | + | + | + | + | + | |||||
| 040702 | + | + | + | + | + | ||||||
| 040703 | + | + | + | + | + | + | |||||
| 0408 | + | + | + | + | + | + | |||||
| 0409 | + | + | + | + | + | + | |||||
| 0410 | + | + | + | + | + | + | |||||
| 0411 | + | + | + | + | + | + | |||||
| 0412 | + | + | + | + | |||||||
| 0413 | + | + | + | + | + | + | |||||
| 0414 | + | + | + | + | + | + | |||||
| 0415 | + | + | + | + | |||||||
| 0416 | + | + | + | + | + | ||||||
| 0417 | + | + | + | + | + | + | |||||
| 0418 | + | + | + | + | |||||||
“+” indicates that the allele produces fluorescent PCR product with the indicated probe
Results
Validation with previously-typed unblinded samples
To optimize the QPCR-SSPP assay and to demonstrate its reliability, 56 samples previously typed using standard methods (PCR-SSO and PCR-SBT) were assayed, without blinding as to previous typing, using both the TaqMan and SYBRgreen I-based assays for DR4 low level resolution. There was 100% concordance between both real-time assays and traditional techniques. 49 of the 56 samples were identified as DR4-positive samples and were assessed for DR4 subtype using the QPCR-SSPP subtyping assay. 39 of these samples were identified as having a single DR4 allele, while 10 were found to have two DR4 alleles. The samples tested contained several representative DR4 alleles, including DRB1*0401, 0402, 0403 (or 0406), 0404, 0405, 0407, 0408, and 0411.
DRB1*0403 alleles (and other DR4 alleles that share the sequence GAG at codon 74, including DRB1*0407 and 0411) were found to react with both the GDR14 and GDR15 probes, though they would have been predicted to generate product only with GDR15. However, while both probes responded, the GDR15 signal was stronger than GDR14 (i.e. the CT for GDR15 was lower than the GDR14- CT). In contrast, DRB1*0401 samples (and other DR4 alleles that share instead the sequence GCG at codon 74 (including DRB1*0404 and 0405) reacted solely with the GDR14 probe. Heterozygous samples that were predicted to respond to both probes GDR14 and 15 (e.g. DRB1*0401/0403), showed a stronger signal for GDR14 than for GDR15. This allowed straight-forward interpretation of true positives, as GDR14 signal was only scored as positive if it appeared with a lower CT than that of GDR15. The limited cross-reactivity in the codon 74 region, between GAG-containing alleles and the GDR14 probe, was likely due to the high G/C content and was not observed with the other subtyping reactions. The subtypes determined by the QPCR-SSPP assay were 100% concordant with previous standard typing methods.
Validation with blinded samples
To further compare the reliability and accuracy of the real-time PCR approach for HLA DR4 genotyping, 44 samples that had been previously typed using standard techniques (PCR-SSO, PCR-SSP, or PCR-SBT) were blinded and analyzed using the QPCR-SSPP rapid assay. Samples were first screened for the presence of DR4 using the DRA control primers and pan-DR4 group-specific amplification. Of 44 samples analyzed, 28 of 28 DR4-positive samples were correctly identified. No false positives were detected. Samples that were identified as DR4-positive were then analyzed for DR4 subtype using the QPCR-SSPP subtyping assay. Of the 28 DR4-positive samples, 25 were found to have a single DR4 allele present, while three samples were found to be heterozygous for two DR4 alleles. Samples represented the DR4 subtypes, DRB1*0401, 0402, 0403, 0404, 0405, and 0407. QPCR-SSPP typing of all samples was concordant with conventional typing approaches.
Determination of copy number of DR4 alleles
Real-time PCR, like traditional PCR, relies on the ability to duplicate the quantity of double stranded DNA with each amplification cycle. Using TaqMan chemistry, a probe, labeled with a reporter fluor and a non-fluorescent quencher, binds to the target template. During the extension reaction, the fluor is cleaved by the 5′ nuclease activity of the polymerase and released. The measured fluorescence is therefore a direct measure of the number of molecules of product. The cycle at which accumulated fluorescence reaches a set threshold level (CT) during the exponential growth phase of the PCR can be measured. A 2-fold difference in starting template quantity will result in a 1 cycle difference to reach this threshold level of fluorescence. If equal quantities of genomic DNA are assayed for DR4, then a sample with two DR4 alleles (e.g. DRB1*0401/0401 or *0401/0404) will display a CT that is 1 cycle earlier than will a sample with a single DR4 allele (DRB1*0401/non-DR4). Since it is difficult to accurately quantitate and pipet precisely the same quantity of sample in multiple PCR reactions, we utilized a reference gene to control for quantity of input DNA. Since we were interested in detecting HLA alleles, we chose another class II gene, DRA, although any invariant gene would work in this role. DRA is an essentially non-polymorphic, diploid gene, shared by all individuals, regardless of HLA type. By simultaneously amplifying DRA, along with pan-DRB1*04, we were able to assess the number of copies of DR4 alleles by comparing the CT at which signal from DR4 appears with the CT for DRA. Since DRA is present in two copies in every individual, it is possible to compare the CT of DR4 product to that of DRA to assess whether an individual carries one or two DR4 alleles. The difference in these CT measurements (delta CT) is proportional to the copy number of DR4 alleles. As shown in Figure 1, the delta CT for DR4 homozygotes or compound heterozygotes (e.g. DRB1*0401/0401 or *0401/0404) are easily distinguished from DR4 “hemizygotes” (e.g. DRB1*0401/non-DR4) (p<0.0001, two-tailed, unpaired t test). The mean delta CT for samples with one copy of DR4 was 1 cycle greater than for samples with two copies of DR4. This corresponds to a two-fold difference in starting quantity per genome equivalent in samples with one or two copies of DR4 alleles. Analysis of both blinded and unblinded previously-typed samples demonstrated that this approach can be used to both accurately and reproducibly assess DR4 copy number.
Fig. 1.
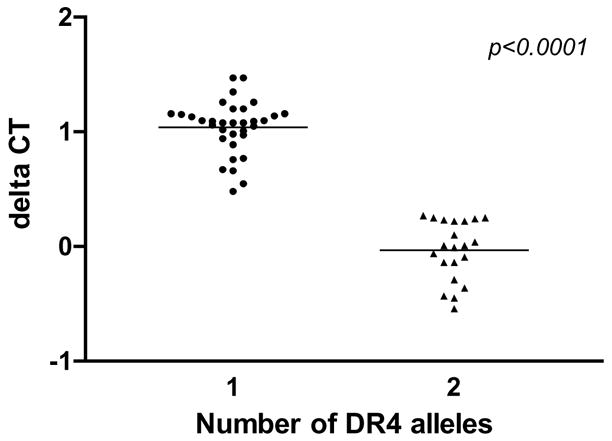
Determination of copy number of DR4 alleles. Genomic DNA from 54 conventionally typed samples was assayed by QPCR-SSPP for DRA and pan-DR4 in a multiplex TaqMan reaction to determine the quantity of DR4 per genomic equivalent. The cycle (CT) during which each sample reached an arbitrary threshold of fluorescence was determined, and the delta CT was calculated as CT DR4 – CT DRA. The mean delta CT for samples with either one or two copies of DR4 alleles is shown; the difference between these means is significant by a two-tailed, unpaired t test.
Reproducibility and assay variation
In developing the QPCR-SSPP assay, a subset of both blinded and unblinded samples were tested in replicate, both on the same assay plate and on separate plates (data not shown). Identification of HLA subtype was found to be equally accurate with replicates as with singlate reactions. Through the use of multiple built-in control elements, including simultaneous amplification of DRA as a control for quality and quantity, pan-DR4 for low resolution typing and dose determination, and the use of pairs of single- and multiple-nucleotide polymorphism-detecting probes for subtype determination, the QPCR-SSPP assay was able to easily distinguish true positives from the rare artifacts that can be observed in PCR. Likewise, for determination of DR4 copy number, the amplification of both the DRA control and pan-DR4 in a single well decreased the problem of pipetting error in dose determination. As shown in Figure 1, samples assayed in different runs demonstrated similar delta CT values. Thus, the use of singlate reactions resulted in increased throughput without a loss of accuracy.
Discussion
The QPCR-SSPP rapid TaqMan-based typing technique presented here allows determination of DR4 subtypes, as well as copy number (DR4 homozygote or heterozygote vs. DR4 hemizygotes) in less than two hours, including setup and analysis, starting with genomic DNA. It is sensitive and specific and gives fewer ambiguous results than other approaches due to the high level of specificity and sensitivity of the TaqMan technology and the decreased dependency on operator interpretation of agarose gel bands or hybridization spots. For rapid low-level resolution of DR4, 96 samples can be screened for pan-DR4 and DRA on a single reaction plate. Subtyping for DR4 can be carried out either simultaneously or subsequent to low-level resolution. In a typical 96 well format, 16 samples can be simultaneously screened and subtyped for DR4. The sample cost is quite low, providing significant savings over current techniques. With the increasing availability of 384-well and fast-block platforms, throughput, time, and cost could be further enhanced. Most of the effort required by the assay is automated and hands-free. The remainder is easily amenable to robotic devices.
This rapid subtyping approach is an extension of traditional methodology, basically an adaptation of PCR-SSO and PCR-SSP typing using a real-time PCR detection instrument. This means that the PCR is done in a sealed system and that no post-PCR manipulation is required. The amplification, hybridization, and detection are all performed simultaneously on the instrument. Further, we have created a simple set of analysis algorithms in Microsoft® Excel that quickly analyzes data output and generates subtyping results in a matter of minutes.
While we have also designed and validated primers for DR4 subtyping using SYBRgreen I with real-time PCR detection (data not shown), the assay was less robust than the TaqMan approach, and we have chosen to focus on the latter here. However, the use of SYBRgreen I for low resolution typing of DR4 and determination of copy number is almost equally effective to the TaqMan assay. The primary advantage of using SYBRgreen I dye is the cost savings by avoiding the need for the synthesis of fluorescently-modified probes. This advantage is offset, however, by decreased throughput, since SYBR assays cannot be run as a multiplex PCR. Further, the resultant increase in the number of wells, and hence the volume of reagents required per sample, offsets both the efficiency and cost-effectiveness of the SYBR approach. Therefore, we favor the TaqMan approach, both for low and high resolution determinations.
Real-time PCR not only allows rapid determination of specific HLA alleles but is also useful for the determination of allele copy numbers. Since disease risk and severity can be affected both by DR4 subtype as well as by the number of DR4 alleles present, this is a relevant marker to assess. In RA, for example, individuals who carry a single copy of either DRB1*0401 or 0404 have a relative risk of approximately 6 for the development of severe erosive disease, compared to 1.0 for the non-DR4 general population. In contrast, individuals homozygous for DRB1*0401 or *0404 carry a relative risk of approximately 15 and those with both DRB1*0401 and 0404 have a relative risk of at least 49 [Nepom et al., 1987; Wordsworth et al., 1992; Nepom et al., 1992; Fries et al., 2002]. Thus, it is useful to be able to assess zygosity as well as determining specific subtypes. Traditionally, determination of homozygosity is made by conventional full typing at the DRB locus, but that can be a costly approach to generate allelic dosage information, especially if identification of non-DR4 alleles is not needed. Additionally, it relies on the absence of a detectable second allele which infers homozygosity of the detected allele. Real-time PCR offers a simple, inexpensive option for determining copy number of DR4 alleles along with subtype. Rather than an inferred result, this is a real measurement of the number of DR4 alleles per genome.
There have been a limited number of recent reports of the use of real-time PCR approaches for low-level resolution of HLA (e.g. [Slateva et al., 2001; Sylvain et al., 2004; Casamitjana et al., 2005]) and one report of a high resolution TaqMan-based typing assay [Tremmel et al., 1999]. In contrast to our study, Tremmel et al. (1999) relied largely on sequence-specific primers to discriminate alleles of DRB1*15 and *16 and incorporated a separate internal control amplification into each reaction. We chose to use group-specific primers in combination with polymorphism-specific probes for allele identification, with a single internal control reaction. Though both approaches have merits, by multiplexing probes for alternate polymorphisms in the same reaction, we were able to significantly reduce the number of wells needed for high resolution typing. Since most DR4 samples are positive for at least one of the probes in each codon region, each well acts both to discriminate alleles and as a simultaneous positive and negative control. At the same time, we were able to design an internal standard that successfully allows determination of DR4 copy number along with allele identification. Our study is the first report of the use of real-time technology for high-resolution HLA typing of DRB1*04.
We have shown here that DR4 subtyping and dose determinations can be accomplished rapidly, accurately, and in a cost-efficient manner. The use of real-time PCR for the detection and differentiation of HLA type is readily adaptable to other DRB alleles, as well as other gene loci. While this approach is less easily applied to the discovery of novel polymorphisms, it is readily adaptable to the development of routine HLA typing at all resolutions and is applicable for rapid screening of large sample numbers. Thus, QPCR-SSPP is an economical and efficient approach for both research and clinical laboratories, and for targeted genetic analyses, it offers a major improvement over existing conventional approaches.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation and The National Institutes of Health. We thank Will Leighty and the BRI Clinical Core Registry for providing genomic DNA samples for validation analyses and Lakshmi Gaur and the Puget Sound Blood Center for reference HLA typing of some of the DNA samples.
Abbreviations
- HLA
Human leukocyte antigen
- MGB
Minor Groove Binder
- PCR
Polymerase chain reaction
- PCR-SBT
Polymerase chain reaction with sequence-based typing
- PCR-SSO
Polymerase chain reaction with sequence specific oligonucleotide probes
- PCR-SSP
Polymerase chain reaction with sequence specific primers
- QPCR-SSPP
Quantitative real-time PCR with sequence specific primers and probes
- RA
Rheumatoid Arthritis
- T1D
Type I diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Casamitjana N, Faner R, Santamaria A, Colobran R, Ribera A, Pujol-Borrell R, Juan M, Palou E. Development of a new HLA-DRB real-time PCR typing method. Hum Immunol. 2005;66:85. doi: 10.1016/j.humimm.2004.08.178. [DOI] [PubMed] [Google Scholar]
- Doherty D, Penzotti JE, Koelle DM, Kwok WW, Lybrand TP, Masewicz S, Nepom GT. Structural basis of specificity and degeneracy of T cell recognition: Pluriallelic restriction of T cell responses to a peptide antigen involves both specific and promiscuous interactions between the T cell receptor, peptide, and HLA-DR. J Immunol. 1998;161:3527. [PubMed] [Google Scholar]
- Fries JF, Wolfe F, Apple R, Erlich H, Bugawan T, Holmes T, Bruce B. HLA-DRB1 genotype associations in 793 white patients from a rheumatoid arthritis inception cohort: frequency, severity, and treatment bias. Arthritis Rheum. 2002;46:2320. doi: 10.1002/art.10485. [DOI] [PubMed] [Google Scholar]
- Gebe JA, Novak EJ, Kwok WW, Farr AG, Nepom GT, Buckner JH. T cell selection and differential activation on structurally related HLA-DR4 ligands. J Immunol. 2001;167:3250. doi: 10.4049/jimmunol.167.6.3250. [DOI] [PubMed] [Google Scholar]
- Maclaran NK, Riley WJ. Inherited susceptibility to autoimmune Addison’s disease is linked to human leukocyte antigens-DR3 and/or DR4, except when associated with type I autoimmune polyglandular syndrome. J Clin Endocrinol Metab. 1986;62:455. doi: 10.1210/jcem-62-3-455. [DOI] [PubMed] [Google Scholar]
- Nepom BS, Nepom GT, Mickelson E, Antonelli P, Hansen JA. Electrophoretic analysis of human HLA-DR antigens from HLA-DR4 homozygous cell lines: Correlation between beta- chain diversity and HLA-D. Proc Natl Acad Sci USA. 1983;80:6962. doi: 10.1073/pnas.80.22.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom GT. The role of the DR4 shared epitope in selection and commitment of autoreactive T cells in rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:305. doi: 10.1016/s0889-857x(05)70203-9. [DOI] [PubMed] [Google Scholar]
- Nepom GT, Erlich H. MHC class II molecules and autoimmunity. Ann Rev Immunol. 1991;9:493. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Nepom GT, Hansen JA, Nepom BS. The molecular basis for HLA class II associations with rheumatoid arthritis. J Clin Immunol. 1987;7:1. doi: 10.1007/BF00915418. [DOI] [PubMed] [Google Scholar]
- Nepom GT, Nepom BS. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen genotyping. In: Nepom GT, editor. Rheumatic Disease Clinics of North America. W.B. Saunders Company; Philadelphia: 1992. pp. 785–792. [PubMed] [Google Scholar]
- Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134. [PMC free article] [PubMed] [Google Scholar]
- Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing. Tissue Antigens. 1992;39:225. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki RK, Bugawan TL, Horn GE, Mullis KB, Erlich H. Analysis of enzymatically amplified beta-globin and HLA- DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Sayer D, Whidborne R, Brestovac B, Timboli F, Witt C, Christiansen F. HLA-DRB1 DNA sequencing based typing: an approach suitable for high throughput typing including unrelated bone marrow registry donors. Tissue Antigens. 2001;57:46. doi: 10.1034/j.1399-0039.2001.057001046.x. [DOI] [PubMed] [Google Scholar]
- Scharf SJ, Freidmann A, Steinman L, Brautbar C, Erlich HA. Specific HLA-DQB and HLA-DRB1 alleles confer susceptibility to pemphigus vulgaris. Proc Natl Acad Sci USA. 1989;86:6215. doi: 10.1073/pnas.86.16.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slateva K, Elsner HA, Albis-Camps M, Blasczyk R. HLA-DRB fluorotyping by dark quenching and automated analysis. Tissue Antigens. 2001;58:250. doi: 10.1034/j.1399-0039.2001.580405.x. [DOI] [PubMed] [Google Scholar]
- Svejgaard A, Ryder LP. HLA genotype distribution and genetic models of insulin-dependent diabetes mellitus. Ann Hum Genet. 1981;45:293. doi: 10.1111/j.1469-1809.1981.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Sylvain K, Aurelie H, Marc M, Christophe R. Rapid screening for HLA-B27 by a TaqMan-PCR assay using sequence-specific primers and a minor groove binder probe, a novel type of TaqMan trade mark probe. J Immunol Methods. 2004;287:179. doi: 10.1016/j.jim.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Tremmel M, Opelz G, Mytilineos J. High-resolution typing for HLA-DRB1*15 amd -DRB1*16 by fluorescence-marked sequence-specific priming (TaqMan assay) Tissue Antigens. 1999;54:508. doi: 10.1034/j.1399-0039.1999.540508.x. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- Wordsworth B, Lanchbury JSS, Sakkas LI, Welsh KI, Panayi GS, Bell JI. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci USA. 1989;86:10049. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth P, Pile KD, Buckley JD, Lanchbury JSS, Ollier B, Lathrop M, Bell JI. HLA heterozygosity contributes to susceptibility to rheumatoid arthritis. Am J Hum Genet. 1992;51:585. [PMC free article] [PubMed] [Google Scholar]
- Yu L, Brewer KW, Gates S, Wu A, Wang T, Babu SR, Gottlieb PA, Freed BM, Noble J, Erlich HA, Rewers MJ, Eisenbarth GS. DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison’s disease. J Clin Endocrinol Metab. 1999;84:328. doi: 10.1210/jcem.84.1.5414. [DOI] [PubMed] [Google Scholar]


