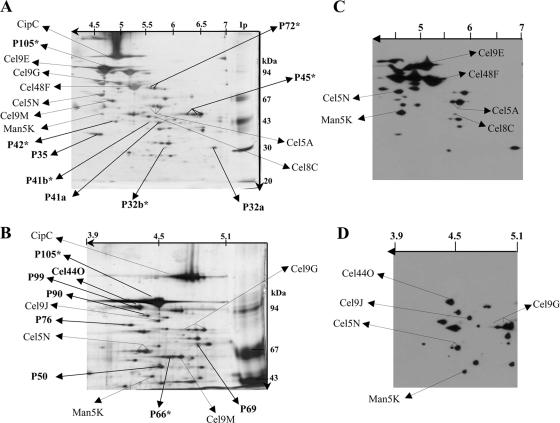
FIG. 1.
Composition of the cellulolytic system of C. cellulolyticum and localization of the dockerin-containing proteins. (A and B) Proteins of the Fc fraction were separated on two-dimensional gels (12% polyacrylamide with IEF at pH 4 to 7 [A] or 8% polyacrylamide with IEF at pH 3.9 to 5.1 [B]) and silver stained. The gel images are oriented with the IEF dimension horizontal and the SDS-PAGE dimension vertical. (C and D) After blotting, proteins separated by 2D electrophoresis on 12% polyacrylamide with IEF at pH 4 to 7 (C) and proteins separated by 2D electrophoresis on 8% polyacrylamide with IEF at pH 3.9 to 5.1 (D) were incubated with biotinylated miniCipC. The positions of known proteins, including CipC, Cel48F, Cel8C, Cel9G, Cel9E, Man5K, Cel9M, Cel5A, Cel5N, Cel9J, and Cel44O are indicated by arrows, as are the positions of some unknown proteins. Each unknown protein, indicated by bold type, is designated “P” followed by a number referring to the molecular mass of the protein. The N-terminal sequences of these proteins and their enzymatic activities as determined by zymogram assays are shown on Table 3. P32b, P41b, P42, P45, P66, P72, and P105 (in bold type with an asterisk) did not interact with biotinylated miniCipC and presumably lack dockerin.