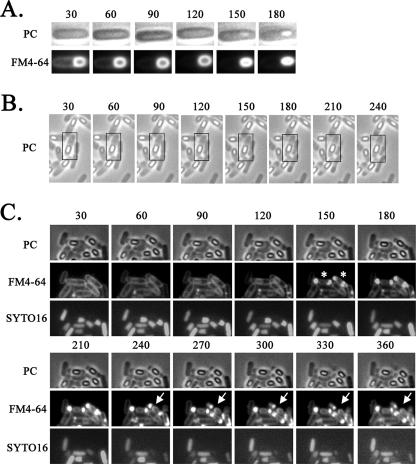
FIG. 3.
Time-lapse microscopy of the mother cell death process. Strain 168 was monitored beginning at 3 h (A), 9 h (B), and 13 h (C) after the start of sporulation. Time-lapse intervals are indicated in minutes from the first observation time. For each time-lapse sequence, phase-contrast (PC) images are shown alone (B) or in the upper row (A and C), corresponding fluorescence images obtained with the membrane-staining dye FM4-64 are shown in the lower row (A) or middle row (C), and images obtained with the DNA-staining dye SYTO16 are shown in the lower row in panel C. SYTO16 fails to stain the developed forespore (bright spore) DNA, but it can stain the mother cell DNA. Time-lapse microscopy was performed essentially as described by Price and Losick (12). Cells were grown and induced to sporulate at 37°C by the resuspension method, in which the resuspension medium contained FM4-64 (final concentration, 0.5 μg/ml) and SYTO16 (final concentration, 0.1 μM). Portions (20 μl) of each culture were mounted on an agarose bed containing FM4-64 and SYTO16. Phase-contrast and fluorescence images were acquired every 30 min over the course of the time-lapse experiments. The time-lapse experiments were carried out at 30°C; representative cell containing the phase-bright forespore moving to the center of the mother cell from one pole of the cell are shown in boxes (B). Rupture II cells at 150 min are indicated with asterisks, and the positions at which to look for leakage of FM4-64 staining are indicated with arrows (C).