Abstract
Amelogenin has chromosome X (AMELX) and Y (AMELY) homologs that can be differentiated based on the length of polymerase chain reaction (PCR) amplification products. In addition to being useful for gender identification, analysis of amelogenin has utility for monitoring bone marrow engraftment in patients after a sex-mismatched bone marrow transplant, characterizing sex chromosome abnormalities, and for forensic purposes for analyzing mixtures of male and female DNA. Here, we describe two brothers in which PCR analysis demonstrated twofold greater AMELY products compared with AMELX products. Karyotype and X/Y fluorescence in situ hybridization analysis demonstrated a single copy of the X and Y chromosomes without any identifiable abnormalities. Oligonucleotide comparative genomic hybridization array analysis demonstrated a duplication of a portion of chromosome Yp that encompassed a region of at least 2.6 Mb but not greater than 4.0 Mb. The amplified region contains the genes AMELY, transducin (β)-like 1 protein Y (TBL1Y), and protein kinase Y (PRKY). To our knowledge, duplication of this region has not previously been reported. The family history is unremarkable, and the brothers are without ap-parent dysmorphic features. Although this and other genetic variants involving AMELY are uncommon, one should use caution when using amelogenin for sex chromosome analysis and bone marrow engraftment analysis.
The Y chromosome is unique in that it is clonally inherited from father to son (with the exception of two small pseudoautosomal regions). The male-specific region of the Y chromosome appears to code for at least 27 distinct proteins or protein families, some of which have been implicated in gonadal sex reversal, Turner syndrome, graft rejection, and spermatogenic failure.1 These syndromes often result from deletions within the Y chromosome or abnormal interchange between chromosomes X and Y.
Many Y-linked genes have X homologs that code for similar protein isoforms. The functional difference (if any) between the X and Y homologs of most of these genes is not well understood. One of the degenerate genes, amelogenin, has X and Y homologs located on Xp22.1-22.3 (AMELX) and Yp11.2 (AMELY). Polymerase chain reaction (PCR) amplification with single primer pairs can differentiate the AMELX and AMELY homologs due to the difference in length of the amplification products. This difference is commonly exploited for gender identification of clinical and forensic specimens. The most commonly used amelogenin PCR-based assay uses a primer pair that spans a 6-bp deletion within intron 1 of the X homolog.2 Amplification of this region has been incorporated into several commercially available multiplex short tandem repeat (STR) reactions. These multiplex assays have utility for identity/forensic and paternity testing purposes. In addition to these qualitative applications, these assays can be used quantitatively by comparing the relative peak heights or peak areas of the amplification products. Some authors have proposed that comparing the AMELX- and AMELY-specific peak heights can have clinical utility for monitoring bone marrow engraftment in patients after a sex-mismatched bone marrow transplant,3 for characterizing sex chromosome abnormalities such as Klinefelter syndrome (XXY),2 and for forensic purposes for quantification of the relative X and Y contribution in mixtures of male and female DNA.4 Here, we describe two brothers who have a constitutional duplication of a portion of the short arm of chromosome Y that was initially identified by amelogenin analysis.
Materials and Methods
DNA Isolation
DNA was isolated from all peripheral blood and bone marrow specimens using the Qiagen EZ1 automated nucleic acid extraction instrument and reagents or QIAamp DNA Mini kit spin columns according to the manufacturer’s instructions (Qiagen, Valencia, CA).
Amelogenin Analysis
PCR amplification was performed using the AmpFlSTR Profiler kit (Applied Biosystems, Foster City, CA) that detects nine microsatellite loci and the amelogenin locus. The amelogenin primer pair amplifies a region of intron 1 that spans a 6-bp deletion in AMELX compared with AMELY.2 Thermal cycling and capillary electrophoresis were performed according to the manufacturer’s instructions. In brief, the PCR conditions were 95°C for 11 minutes; followed by 28 cycles of 94°C for 1 minute, 59°C for 1 minute, and 72°C for 1 minute; followed by 60°C for 45 minutes. After amplification, 1 μl of multiplex PCR product was mixed with 9 μl of deionized formamide/GeneScan-500 [ROX] size standard solution (Applied Biosystems). Samples were denatured at 95°C for 2 minutes and placed on ice for at least 1 minute before analysis on the ABI 3100 capillary electrophoresis instrument (Applied Biosystems).
Amplification of a region spanning exon and intron 4 of amelogenin was performed using a previously described primer set that spans a 3-bp deletion in AMELX compared with AMELY.5 PCR amplification was performed in 25-μl reactions containing 200 μmol/L of each dNTP, 0.8 μmol/L of each primer, and 1.25 U of Taq Gold polymerase (Applied Biosystems). Primers were 5′-FAM-CCCTTTGAAGTGGTACCAGAGCA-3′ (AMEL-U1) and 5′-GCATGCCTAATATTTTCAGGGAATA-3′ (AMEL-D1). Reactions were thermal cycled as follows: one cycle of 95°C for 9 minutes; followed by 25 cycles of 95°C for 30 seconds, 57°C for 30 seconds, and 72°C for 30 seconds; followed by one cycle of 72°C for 7 minutes. Products were analyzed by capillary electrophoresis as described above.
Analysis of Protein Kinase X and Y Genes (PRKX and PRKY) and Zinc Finger X and Y Genes (ZFX and ZFY)
A PCR was designed to amplify exon 8 of the PRKX and PRKY genes. The primer set spans a 3-bp deletion in PRKY, producing a PRKY product that is 3 bases shorter than the PRKX product. PCR amplification was performed in 25-μl reactions containing 200 μmol/L of each dNTP, 0.8 μmol/L of each primer, and 1.25 U of Taq Gold polymerase (Applied Biosystems). Primers were 5′-FAM-TTTTGTTTCTTTCTGTCCATACTTAAAG-3′ (PRK-F) and 5′-TCCCAAACCACTCAACTG-3′ (PRK-R). Reactions were subjected to thermal cycling as follows: one cycle of 95°C for 9 minutes; followed by 25 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; followed by one cycle of 72°C for 7 minutes. Products were analyzed by capillary electrophoresis as described above.
A PCR assay was designed to amplify exon 3 of the ZFX and ZFY genes. The primer set spans a 3-bp deletion in ZFX, producing a ZFX product that is 3 bases shorter than the ZFY product. PCR amplification was performed in 25-μl reactions containing 200 μmol/L of each dNTP, 0.8 μmol/L of each primer, and 1.25 U of Taq Gold polymerase (Applied Biosystems). Primers were 5′-FAM-TGTGCATAACTTTGTTCCTGATG-3′ (ZF-F) and 5′-AGCACTTGCTCAGGAATGATG-3′ (ZF-R). Reactions were subjected to thermal cycling as follows: one cycle of 95°C for 9 minutes; followed by 28 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; followed by one cycle of 72°C for 7 minutes. Products were analyzed by capillary electrophoresis as described above.
Karyotype and XY Fluorescence in Situ Hybridization (FISH)
The G-banded metaphase karyotype was obtained from B-cell stimulated and unstimulated cells from a bone marrow aspirate of the proband at time of diagnosis of his lymphoma and from phytohemagglutinin-stimulated lymphocytes 3 years after bone marrow transplant, using standard techniques. Centromere enumeration probe X α satellite and centromere enumeration probe Y satellite III DNA FISH probes (Vysis/Abbott Molecular, Des Plaines, IL) were used to evaluate interphase and metaphase cells from the phytohemagglutinin-stimulated lymphocytes following the manufacturer’s protocol.
High-Density Array Comparative Genomic Hybridization
A custom 2 × 105,000 high-density oligonucleotide array was designed using eArray v4.5 software (Agilent Technologies, Santa Clara, CA). Each array contained 4554 control oligonucleotide probes, 18,148 probes on chromosome Y, and 82,298 probes on chromosome X. Comparative genomic hybridization (CGH) was performed by Agilent Technologies according to standard protocol. In brief, genomic DNA (0.5 to 1.0 mg) and male reference DNA (Promega Corporation, Madison, WI) were digested with AluI and RsaI (Promega) at 37°C for 2 hours and then heat-inactivated at 65°C for 20 minutes. The reaction was then used directly as a template for a genomic DNA labeling reaction using random primers and the exo-Klenow fragment in the Agilent Genomic Labeling Kit Plus (Agilent Technologies). Test and reference DNA samples were labeled with either Cy3- or Cy5-dUTP according to the manufacturer’s protocol for 2 hours at 37°C. After heat inactivation at 65°C for 10 minutes, the labeled DNA products were purified using Microcon YM-30 filtration devices (Millipore, Inc., Bedford, MA) and brought to a volume of 80.5 μl with nuclease-free water. The DNA yield and level of dye incorporation were then measured using the ND-1000 spectrophotometer. Appropriate Cy5 and Cy3-labeled DNA sample pairs were combined and mixed with human Cot-1 DNA (Invitrogen, Carlsbad, CA), Agilent 10X Blocking Agent, and Agilent 2X Hybridization Buffer. Samples were heated at 95°C for 3 minutes, incubated for 30 minutes at 37°C, and then hybridized to Agilent custom 2 × 105,000 Human Genome CGH microarrays using Agilent SureHyb chambers. The hybridization chambers were then placed in a 65°C rotisserie oven and rotated at 20 rpm for 40 hours. The arrays were washed and dried according to the procedures described in Agilent Oligonucleotide Array-Based CGH for Genomic DNA Analysis protocol version 4. Microarray slides were scanned immediately using an Agilent microarray scanner. Data were extracted using the Feature Extraction software v9.1.
Results and Discussion
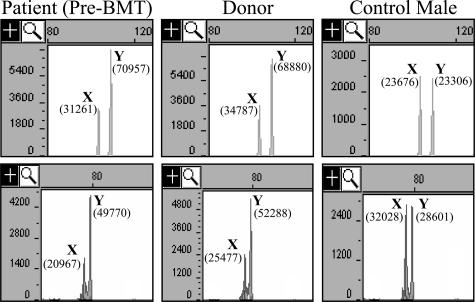
The patient is a 39-year-old white male who was diagnosed with diffuse large B-cell non-Hodgkin’s lymphoma with a bulky mediastinal mass approximately 6 years ago. He was treated with standard doses of chemotherapy and radiation therapy and then underwent an allogeneic bone marrow transplant with his brother as the donor. As part of routine workup before the patient’s bone marrow transplant, peripheral blood specimens from the patient and his donor were evaluated using the AmpFlSTR Profiler kit (Applied Biosystems), which detects nine STR loci and the amelogenin locus. This evaluation of polymorphic differences between the patient and donor allows for quantification of chimerism after transplantation. Analysis of the amelogenin locus of both the patient and his donor identified the AMELX- and AMELY-specific peaks; however, the AMELY peak was approximately twofold greater in height and area than the AMELX peak in both individuals (Figure 1). The average Y:X ratio from at least two replicates was 1.9:1 for the donor and 2.0:1 for the patient before transplant. Since the bone marrow transplantation, the patient has been evaluated for chimerism three times. The STR results of two informative loci were consistent with the patient having 100% donor engraftment. Consistent with the donor’s amelogenin pattern before transplant, in each of the three follow-up analyses, the average amelogenin Y:X ratio (from at least two replicates each) was approximately 1.9:1. Review of 102 cases of males run in our laboratory showed an average amelogenin Y:X ratio of 0.96:1 with SD of 0.09. The highest Y:X ratio seen in this group of males was 1.2:1. Thus the Y:X ratio seen for both the patient and his brother (donor) was more than 9 SD greater than the average Y:X ratio observed in our laboratory. These results suggested that both brothers had an extra copy of the AMELY gene.
Figure 1.
Capillary electrophoresis analysis of amplification of intron 1 (top) and exon/intron 4 (bottom) of amelogenin. Horizontal axis is size in bases. Vertical axis is fluorescence intensity. Shown are results from the patient before bone marrow transplant (pre-BMT), donor, and a representative normal male. AMELX- and AMELY-specific peaks are identified by X and Y, respectively. The area of each peak is shown in parentheses.
To confirm the apparent extra copy of AMELY, we used an additional amelogenin primer set approximately 700 bp downstream of the AmpFlSTR Profiler primer set, which spans a 3-bp deletion in AMELX compared with AMELY. Amplification of 10 normal males demonstrated an average Y/X ratio of 0.98:1 with a SD of 0.046. Amplification of the patient and donor specimens demonstrated Y/X ratios of 2.1:1 and 2.2:1, respectively (Figure 1). These results support the conclusion that both brothers carry an additional copy of the AMELY gene.
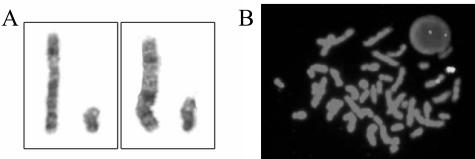
It has been suggested that quantitative analysis of amelogenin can identify sex chromosome aneuploidy. For example, Sullivan et al2 demonstrated that DNA from XYY individuals gave an AMELY/AMELX ratio of 1.8:1, whereas individuals with XXY genotype gave a 1:1.8 ratio. In this case, however, metaphase analysis of 30 cells from the bone marrow of the patient before his bone marrow transplant found a normal male karyotype of 46,XY in all cells analyzed. Karyotype and X/Y FISH analysis of a specimen after transplant from the patient demonstrated a single copy of the X and Y chromosomes without any identifiable abnormalities (Figure 2). Thus the extra copy of AMELY identified in these brothers was not due to an extra copy of the Y chromosome.
Figure 2.
Partial karyotype (A) and XY FISH (B) on metaphase and interphase cells demonstrated a single copy of chromosomes X and Y without identifiable abnormalities.
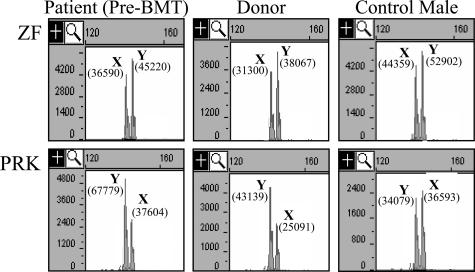
We then analyzed two additional genes on chromosome Yp that have X and Y homologs that can be distinguished by PCR product size. The PRK genes have X and Y homologs located on Xp22.3 (PRKX) and Yp11.2 (PRKY), respectively. The PRKY gene is located approximately 0.35 Mb centromeric to AMELY. To differentiate PRKX and PRKY, we designed a PCR to encompass a 3-bp deletion in PRKY compared with PRKX. Amplification of 10 normal males demonstrated an average PRKY/PRKX ratio of 0.87:1, with a SD of 0.026. Amplification of the patient and donor specimens demonstrated PRKY/PRKX ratios of approximately 1.7:1, which is almost exactly twofold greater than the average PRKY/PRKX ratio seen in our normal male population (Figure 3). These data were similar to the amelogenin results.
Figure 3.
Capillary electrophoresis analysis of amplified products from genes ZF (top) and PRK (bottom). Horizontal axis is size in bases. Vertical axis is fluorescence intensity. Shown are results from the patient before transplant, the donor, and a representative normal male. The X and Y homologs of each gene are identified by X and Y, respectively. The area of each peak is shown in parentheses.
The ZF genes have X and Y homologs located at Xp22.1 (ZFX) and Yp11.2 (ZFY), respectively. ZFY is located approximately 3.9 Mb telomeric to AMELY. To differentiate ZFX and ZFY, we designed a PCR to amplify exon 3 of ZF, encompassing a 3-bp deletion in ZFX compared with ZFY. Amplification of 10 normal males demonstrated an average ZFY/ZFX ratio of 1.21:1, with a SD of 0.084. Amplification of the patient and donor specimens demonstrated ZFY/ZFX ratios that were very similar to that of the normal population (1.25:1 and 1.31:1, respectively, Figure 3). Taken together, the data suggested that both brothers carried a duplication of a portion of chromosome Yp that included PRKY and AMELY, but not ZFY.
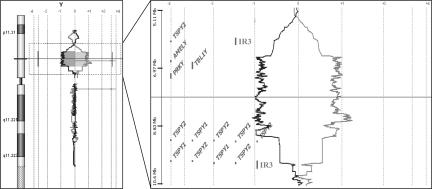
To confirm the apparent duplication on chromosome Yp, we performed CGH using a custom-designed high-density oligonucleotide array. The patient’s genomic DNA (pre-transplant) and a male reference DNA were labeled with either Cy3 or Cy5 and then hybridized to a custom-designed array with 18,148 probes on chromosome Y and 82,298 probes on chromosome X. The experiment was performed twice, using the test and control DNA labeled in opposite colors (dye flip). Results of chromosome X analysis showed no copy number changes (data not shown). Analysis of chromosome Y demonstrated a duplication in chromosome Yp that encompassed a region of at least 2.6 Mb but not greater than 4.0 Mb (Figure 4). To our knowledge, duplication of this region has not previously been reported. We are unable to determine the exact breakpoints for this duplication with high confidence using CGH because this region is flanked by repetitive sequences, and therefore the number and density of CGH probes in the flanking regions is low. There are previous reports describing constitutional deletions in this region.6,7,8,9,10,11 The exact breakpoint for most of these deletions have not been determined, but many of these deletions appear to be greater than 1 Mb, and one was reported to be 2.5 Mb. These deletions significantly overlap the region we show to be duplicated. In addition, Repping et al12 have recently reported inversion of a similar region of chromosome Yp in 16 of 47 Y chromosomes that represented branches of a worldwide genealogical tree. The 3.6-Mb inversion they identified is flanked by nonpalindromic inverted repeats (IR3), which flank the duplication identified here (Figure 4). Repping et al12 suggested that the inversions that they identified originated via ectopic homologous recombination between the IR3 repeats. Thus, this region on chromosome arm Yp seems to be a hotspot for mutation/rearrangement, allowing for deletion, inversion, and now duplication of this region.
Figure 4.
Oligonucleotide CGH analysis of the Y chromosome of the patient before transplant. The gray line to the right represents the patient/control fluorescence intensity ratios (patient labeled in Cy5, control in Cy3). The dark line to the left represents fluorescent intensities obtained from a second hybridization in which the dyes have been reversed (control/patient). An expanded view of the duplicated region is shown to the right, demonstrating the genes in this region.
The CGH data demonstrate that the amplified region contains the three genes AMELY, PRKY, and Transducin (β)-like 1 protein Y (TBL1Y) and may contain all or part of the testis-specific protein Y (TSPY) gene family. The three genes known to be duplicated are single-copy genes located within the X-degenerate sequence of chromosome Y, and all have X-linked homologs, AMELX, PRKX, and TBL1X, respectively. PRKX and PRKY are members of the cAMP-dependent serine threonine protein kinase gene family and are ubiquitously expressed.13,14 Although PRKX has been implicated in renal epithelial cell migration and morphogenesis,15 and in granulocyte/macrophage lineage differentiation,16,17 the function of PRKY is essentially unknown. The TBL1 gene family has at least three members: TBL1X, TBL1Y, and TBL1R, an autosomal homolog located on chromosome 3. Partial deletion of TBL1X appears to be related to X-linked recessive late-onset sensorineural deafness.18 TBL1Y differs from other members of the family in tissue expression pattern and function and cannot completely compensate for a lack of TBLX.19 Therefore TBL1Y may play other, yet unknown, unique roles in males.
AMELX and AMELY are highly conserved extracellular matrix proteins expressed during tooth enamel development.20 AMELX is expressed at levels tenfold higher than AMELY, and mutations in AMELX result in the congenital enamel defect termed amelogenesis imperfecta.21,22 In contrast, deletions of AMELY have been reported in apparently healthy males.6,7,8,9,10 These deletions occur more frequently in certain ethnic populations and may reflect a common ancestor for at least some of these chromosomes.11 Some of the deletions also appear to include deletion of TBL1Y and PRKY.6,10
The TSPY gene family is based on a 20.4-kb repeat unit and contains approximately 35 copies of TSPY genes in a highly regular tandem arrangement.1 TSPY expression is limited to the testis where it is believed to function as a proliferation factor during spermatogenesis, although its exact biological function remains to be elucidated. TSPY has been implicated as a putative oncogene involved in testicular tumorigenesis.23,24 Because of the repetitive nature of this region, the oligonucleotide probe density in this region is low, resulting in uncertainty as to whether all or part of the TSPY gene family is included in the duplicated region.
The duplication described here occurs in two brothers of Italian descent and is presumed to be inherited from their father. The family history is unremarkable, and the brothers are without apparent dysmorphic features. Specifically, there is no history of male-related infertility, cancer (other than the patient), or teeth abnormalities (given the involvement of the amelogenin gene). The lack of a phenotype associated with this duplication may explain why this genetic variant has not been previously identified. We have analyzed more than 700 males using the AmpFlSTR Profiler reaction as part of bone marrow transplant monitoring and have not previously observed an unequal Y/X ratio in an apparently healthy male, suggesting that this duplication is a relatively rare event.
An additional copy of the entire Y chromosome (XYY syndrome) occurs in approximately 1 in 1000 newborn males. Although the clinical phenotype is variable, many XYY individuals are phenotypically normal and are never diagnosed.25 Therefore, the lack of phenotype in these brothers with a duplication of a portion of chromosome Yp may not be surprising. Interestingly, it has been suggested that a constitutional XYY genotype may be associated with an increased risk for leukemia and lymphoma in such individuals, but this is tenuous (previously reviewed26). The finding of a male with lymphoma and a duplication of only a portion of chromosome Yp may be of interest to this ongoing controversy.
In conclusion, we believe that this is the first report describing a constitutional duplication in the region of chromosome Yp that includes AMELY, PRKY, and TBL1Y. This region appears to be a hotspot for genetic events, which can result in deletion, inversion, or duplication of this region. From the literature and our observation, it appears that these events do not result in an appreciable phenotype. However, the resulting copy number changes can result in incorrect interpretation of amelogenin analysis. In this case, duplication of AMELY could have lead to sex chromosome interpretation as XYY. Deletions in the region containing AMELY result in such individuals being falsely genotyped as female.6,7,8 Both duplication and deletion of AMELY can also result in erroneous bone marrow engraftment analysis results. We therefore recommend caution when using amelogenin analysis for sex chromosome analysis or bone marrow engraftment analysis. Sex chromosome abnormalities identified by amelogenin analysis should be confirmed by XY FISH and/or karyotyping. For bone marrow engraftment analysis, the amelogenin pattern before transplant of both the patient and donor should be analyzed closely. As recommended previously,27 at least two loci should be used for all analyses after transplant.
Acknowledgments
We thank Mr. Will Ferguson for technical assistance with the CGH data and Drs. Denise Batista and Gail Stetton for valuable discussions and comments.
References
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Sullivan KM, Mannucci A, Kimpton CP, Gill P. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques. 1993;15:636–638, 640–641. [PubMed] [Google Scholar]
- Toren A, Rechavi G, Nagler A. Minimal residual disease post-bone marrow transplantation for hemato-oncological diseases. Stem Cells. 1996;14:300–311. doi: 10.1002/stem.140300. [DOI] [PubMed] [Google Scholar]
- Mannucci A, Sullivan KM, Ivanov PL, Gill P. Forensic application of a rapid and quantitative DNA sex test by amplification of the X-Y homologous gene amelogenin. Int J Legal Med. 1994;106:190–193. doi: 10.1007/BF01371335. [DOI] [PubMed] [Google Scholar]
- Haas-Rochholz H, Weiler G. Additional primer sets for an amelogenin gene PCR-based DNA-sex test. Int J Legal Med. 1997;110:312–315. doi: 10.1007/s004140050094. [DOI] [PubMed] [Google Scholar]
- Santos FR, Pandya A, Tyler-Smith C. Reliability of DNA-based sex tests. Nat Genet. 1998;18:103. doi: 10.1038/ng0298-103. [DOI] [PubMed] [Google Scholar]
- Thangaraj K, Reddy AG, Singh L. Is the amelogenin gene reliable for gender identification in forensic casework and prenatal diagnosis? Int J Legal Med. 2002;116:121–123. doi: 10.1007/s00414-001-0262-y. [DOI] [PubMed] [Google Scholar]
- Steinlechner M, Berger B, Niederstatter H, Parson W. Rare failures in the amelogenin sex test. Int J Legal Med. 2002;116:117–120. doi: 10.1007/s00414-001-0264-9. [DOI] [PubMed] [Google Scholar]
- Chang YM, Burgoyne LA, Both K. Higher failures of amelogenin sex test in an Indian population group. J Forensic Sci. 2003;48:1309–1313. [PubMed] [Google Scholar]
- Lattanzi W, Di Giacomo MC, Lenato GM, Chimienti G, Voglino G, Resta N, Pepe G, Guanti G. A large interstitial deletion encompassing the amelogenin gene on the short arm of the Y chromosome. Hum Genet. 2005;116:395–401. doi: 10.1007/s00439-004-1238-z. [DOI] [PubMed] [Google Scholar]
- Chang YM, Perumal R, Keat PY, Yong RY, Kuehn DL, Burgoyne L. A distinct Y-STR haplotype for Amelogenin negative males characterized by a large Y(p) 11.2 (DYS458-MSY1-AMEL-Y) deletion. Forensic Sci Int. 2007;166:115–120. doi: 10.1016/j.forsciint.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Repping S, van Daalen SK, Brown LG, Korver CM, Lange J, Marszalek JD, Pyntikova T, van der Veen F, Skaletsky H, Page DC, Rozen S. High mutation rates have driven extensive structural polymorphism among human Y chromosomes. Nat Genet. 2006;38:463–467. doi: 10.1038/ng1754. [DOI] [PubMed] [Google Scholar]
- Klink A, Schiebel K, Winkelmann M, Rao E, Horsthemke B, Ludecke HJ, Claussen U, Scherer G, Rappold G. The human protein kinase gene PKX1 on Xp22.3 displays Xp/Yp homology and is a site of chromosomal instability. Hum Mol Genet. 1995;4:869–878. doi: 10.1093/hmg/4.5.869. [DOI] [PubMed] [Google Scholar]
- Schiebel K, Mertz A, Winkelmann M, Glaser B, Schempp W, Rappold G. FISH localization of the human Y-homolog of protein kinase PRKX (PRKY) to Yp11.2 and two pseudogenes to 15q26 and Xq12→q13. Cytogenet Cell Genet. 1997;76:49–52. doi: 10.1159/000134514. [DOI] [PubMed] [Google Scholar]
- Li X, Li HP, Amsler K, Hyink D, Wilson PD, Burrow CR. PRKX, a phylogenetically and functionally distinct cAMP-dependent protein kinase, activates renal epithelial cell migration and morphogenesis. Proc Natl Acad Sci USA. 2002;99:9260–9265. [Google Scholar]
- Semizarov D, Glesne D, Laouar A, Schiebel K, Huberman E. A lineage-specific protein kinase crucial for myeloid maturation. Proc Natl Acad Sci USA. 1998;95:15412–15417. doi: 10.1073/pnas.95.26.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesne D, Huberman E. Smad6 is a protein kinase X phosphorylation substrate and is required for HL-60 cell differentiation. Oncogene. 2006;25:4086–4098. doi: 10.1038/sj.onc.1209436. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Ramesar RS, Caciotti B, Winship IM, De Grandi A, Riboni M, Townes PL, Beighton P, Ballabio A, Borsani G. X-linked late-onset sensorineural deafness caused by a deletion involving OA1 and a novel gene containing WD-40 repeats. Am J Hum Genet. 1999;64:1604–1616. doi: 10.1086/302408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HT, Shinka T, Kinoshita K, Sato Y, Umeno M, Chen G, Tsuji K, Unemi Y, Yang XJ, Iwamoto T, Nakahori Y. Molecular analysis of TBL1Y, a Y-linked homologue of TBL1X related with X-linked late-onset sensorineural deafness. J Hum Genet. 2005;50:175–181. doi: 10.1007/s10038-005-0237-9. [DOI] [PubMed] [Google Scholar]
- Nakahori Y, Takenaka O, Nakagome Y. A human X-Y homologous region encodes “amelogenin.”. Genomics. 1991;9:264–269. doi: 10.1016/0888-7543(91)90251-9. [DOI] [PubMed] [Google Scholar]
- Chen E, Piddington R, Decker S, Park J, Yuan ZA, Abrams WR, Rosenbloom J, Feldman G, Gibson CW. Regulation of amelogenin gene expression during tooth development. Dev Dyn. 1994;199:189–198. doi: 10.1002/aja.1001990304. [DOI] [PubMed] [Google Scholar]
- Lagerström M, Dahl N, Nakahori Y, Nakagome Y, Backman B, Landegren U, Pettersson U. A deletion in the amelogenin gene (AMG) causes X-linked amelogenesis imperfecta (AIH1). Genomics. 1991;10:971–975. doi: 10.1016/0888-7543(91)90187-j. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Reijo R, Page DC, Disteche CM. Gonadoblastoma: molecular definition of the susceptibility region on the Y chromosome. Am J Hum Genet. 1995;57:1400–1407. [PMC free article] [PubMed] [Google Scholar]
- Lau Y, Chou P, Iezzoni J, Alonzo J, Komuves L. Expression of a candidate gene for the gonadoblastoma locus in gonadoblastoma and testicular seminoma. Cytogenet Cell Genet. 2000;91:160–164. doi: 10.1159/000056838. [DOI] [PubMed] [Google Scholar]
- Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17:363–368. doi: 10.1002/(sici)1097-0223(199704)17:4<363::aid-pd79>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Limacher JM, Girard-Lemaire F, Jeandidier E, Chenard-Neu MP, Kassem M, Flori E, Bergerat JP. Gastrointestinal stromal tumor in an XYY/XY male. Cancer Genet Cytogenet. 2002;133:152–155. doi: 10.1016/s0165-4608(01)00566-0. [DOI] [PubMed] [Google Scholar]
- Swierczynski SL, Hafez MJ, Philips J, Higman MA, Berg KD, Murphy KM. Bone marrow engraftment analysis after granulocyte transfusion. J Mol Diagn. 2005;7:422–426. doi: 10.1016/S1525-1578(10)60572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]