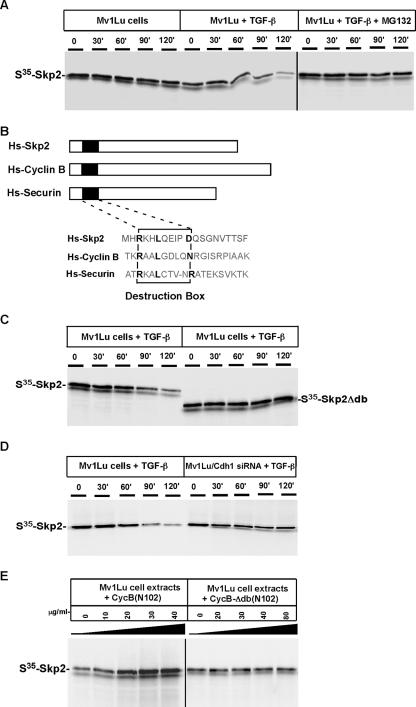
FIG. 3.
Degradation of Skp2 induced by TGF-β is mediated by Cdh1-APC. (A) Skp2 degradation is induced by TGF-β stimulation. Mv1Lu cells were treated (or not treated) with 100 pM TGF-β for 60 min, and extracts were prepared. 35S-labeled in vitro translated Skp2 was added to the extracts and supplemented with protein degradation cocktail. Aliquots were removed at the indicated times and resolved by SDS-PAGE. Skp2 degradation was measured by autoradiography. (B) Alignment of Skp2 with known APC substrates, cyclin B, and securin. Similar to other substrates of APC, Skp2 contains a conserved destruction box [RXXLXXX(N/D)] at its NH2 terminus. (C) Destruction box is required to mediate TGF-β-induced Skp2 degradation. Wild-type and mutant Skp2 proteins lacking the destruction box were incubated in TGF-β-stimulated extracts. (D) Depletion of Cdh1 blocks Skp2 degradation in TGF-β-stimulated extracts. Skp2 was incubated with the extracts prepared from wild-type Mv1Lu or Cdh1 siRNA cells. (E) Degradation of Skp2 is blocked by the addition of a destruction box-containing peptide. NH2-terminal fragments (amino acids 1 to 102) of Xenopus cyclin B or a control fragment lacking the destruction box [CycB-Δdb (N102)] were added to the TGF-β-stimulated extract and 35S-labeled in vitro translated Skp2 at the indicated concentrations. After incubation for 90 min at room temperature, samples were analyzed by SDS-PAGE and autoradiography.