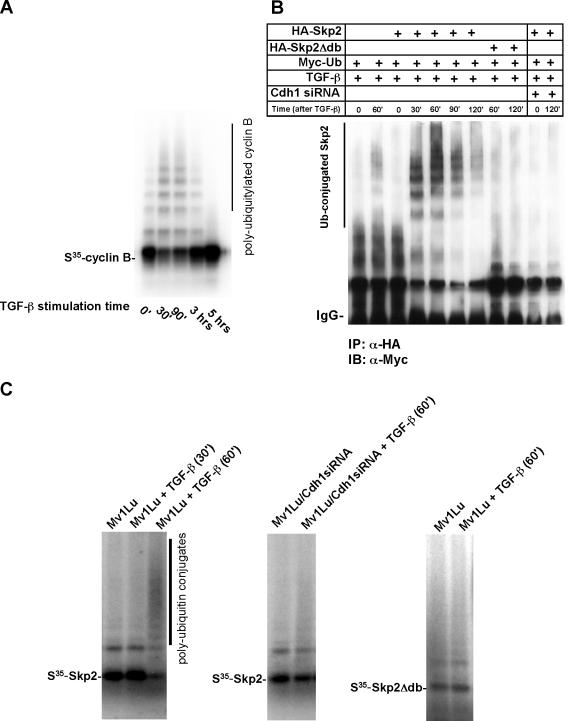
FIG. 4.
Skp2 is ubiquitylated in vivo and in a purified system. (A) Activity of APC is enhanced following stimulation by TGF-β. Mv1Lu cells were stimulated with TGF-β and harvested at different times as indicated. APC was subsequently purified from the cell lysate using anti-Cdc27 antibody coupled to protein A beads and was subjected to ubiquitylation mixture for a ubiquitylation assay. 35S-labeled in vitro translated cyclin B was used as a putative substrate for APC activity analysis. Polyubiquitin conjugates of cyclin B were measured by autoradiography. (B) Skp2 is polyubiquitylated in vivo. Myc-tagged ubiquitin and HA-tagged wild-type Skp2 or mutant Skp2 lacking the destruction box were cotransfected into wild-type or Cdh1 siRNA Mv1Lu cells. Cells were treated with TGF-β and harvested at the indicated times. Skp2 complex was immuno-purified by anti-HA antibody. The polyubiquitin-conjugated Skp2 was detected by immunoblotting using anti-Myc antibody. (C) Recapitulation of Skp2 ubiquitylation in a purified system. Mv1Lu cells were stimulated with TGF-β and harvested at different times as indicated. APC was purified from the cell lysate using anti-Cdc27 antibody coupled to protein A beads and was subjected to a ubiquitylation mixture for the ubiquitylation assay. Polyubiquitin conjugates of Skp2 were measured by autoradiography. Depletion of Cdh1 attenuates TGF-β induced Skp2 ubiquitylation. In addition, disruption of the destruction box blocks TGF-β-induced Skp2 ubiquitylation. IP, immunoprecipitation; IB, immunoblotting; α, anti.