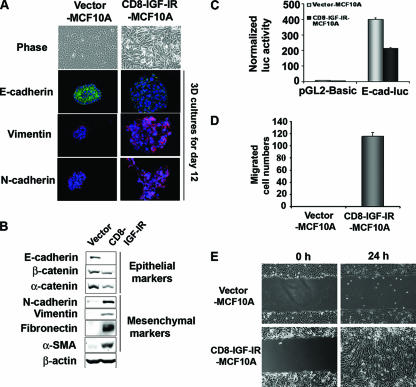
FIG. 4.
EMT was induced in CD8-IGF-IR-MCF10A cells. (A) The morphologies of MCF10A cells expressing either the control vector or CD8-IGF-IR were revealed by phase-contrast microscopy (×20 magnification) at day 3. Vector-MCF10A and CD8-IGF-IR-MCF10A cells were cultured on Matrigel for 12 days and then stained with antibodies to E-cadherin (green), vimentin (red), or N-cadherin (red). The nuclei (blue) were labeled with TOPRO-3 and visualized by confocal microscopy with ×40 magnification. (B) Levels of epithelial proteins, including E-cadherin, β-catenin, and α-catenin, and mesenchymal proteins, including N-cadherin, vimentin, fibronectin, and α-SMA, in vector-MCF10A and CD8-IGF-IR-MCF10A cells were examined by immunoblotting. β-Actin was used as a loading control. (C) The activity of a 2-kb fragment of the E-cadherin promoter upstream of a luciferase reporter (E-cad-luc) was compared to that of the control plasmid (pGL2-basic) by transient transfection. The luciferase (luc) activity was normalized to the cotransfected β-galactosidase activity. The error bars represent the standard errors of the means. (D) Results of the transwell migration assay in which vector-MCF10A and CD8-IGF-IR-MCF10A cells were induced to migrate toward growth media. The migrated cells that passed through the membrane to the lower surface were counted in four different microscopic fields at ×10 magnification. (E) Confluent vector-MCF10A and CD8-IGF-IR-MCF10A cells were scratched and photographs were taken immediately (0 h) and 24 h postscratch at ×10 magnification.