FIG. 3.
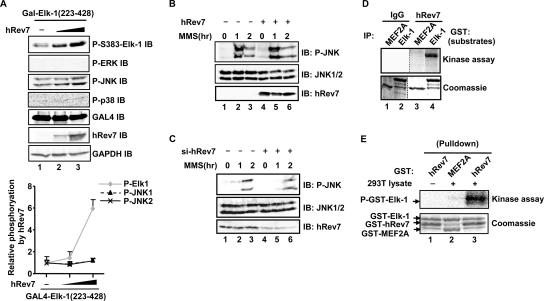
hRev7 enhances Elk-1 phosphorylation in vivo and in vitro. (A) hRev7 induces Elk-1 phosphorylation in vivo. 293T cells were transfected with 0.2 μg GAL4-Elk-1(223-428) and 0 (−), 0.2, or 1 μg hRev7 expression plasmid. Levels of phosphorylation and total protein were determined by immunoblotting (IB) using the appropriate standard or phospho-specific antibodies as indicated (P-S383-Elk-1, phospho-S383 Elk-1; P-ERK, phospho-ERK.). The graph shown below is the average level of Elk-1 and JNK phosphorylation (relative to the levels in the absence of hRev7, taken as 1) normalized for GAPDH levels, from three independent experiments (presented as mean ± standard deviation [error bar]). (B and C) hRev7 overexpression and depletion does not alter JNK activation. 293T cells were transfected (+) with 2.5 μg hRev7 expression plasmid (B) or siRNA duplexes against hRev7 (si-hRev7) (C) where indicated and treated with MMS for 0, 1, or 2 h. Levels of JNK phosphorylation and total protein were determined by immunoblotting (IB) using the appropriate standard or phospho-specific antibodies as indicated. (D) Immune complex in vitro kinase assays with kinases isolated by immunoprecipitation (IP) with either immunoglobulin G (IgG) or hRev7 antibodies from 293T cells transfected with hRev7 expression vectors. Purified GST-Elk-1 or GST-MEF2A were used as substrates. Phosphorylation levels were revealed by phosphorimaging (top blot) and total protein levels by staining with Coomassie blue (bottom blot). (E) GST pulldown and kinase assay. GST-hRev7 or GST-MEF2A beads were used to pull down kinases from 293T lysates where indicated, and the precipitated complexes were used to phosphorylate GST-Elk-1(223-428). Reactions were analyzed as described above for panel D.