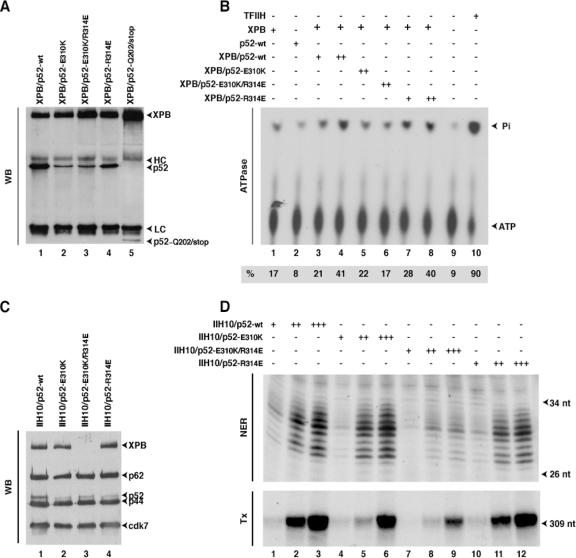
FIG. 5.
Mutations in HP52 cause low stimulation of XPB ATPase activity and NER defects. (A) Recombinant XPB and mutant HP52 expressed in insect cells were immunoprecipitated with an anti-XPB antibody and analyzed by Western blotting (WB). The positions of XPB, HP52, the truncated p52-Q202/stop, and the heavy (HC) and light (LC) immunoglobulin chains are indicated. (B) Wt XPB, either alone or in combination with the p52 mutants, was immunoprecipitated with Ab-XPB antibody immobilized on agarose beads. The immobilized protein complexes were washed with 0.4 M KCl and tested in an ATPase assay. TFIIH was used as a control. Quantifications of the inorganic phosphate (Pi) release and ATP were done with a Bio-imaging analyzer, and the ratio presented at the bottom of the figure shows the percentage of the released Pi. (C) Immunopurified recombinant TFIIHs (as indicated at the top of the blot) from insect cell extracts containing all the TFIIH subunits, including either wt HP52 or mutant HP52, were subjected to Western blot analysis using antibodies against XPB, p62, p52, or Cdk7. The TFIIH subunits are indicated by arrowheads. (D) Fifty, 100, and 200 ng of the various IIH10 complexes were tested in a dual-incision assay (NER) containing the recombinant XPC-HR23b, XPA, RPA, XPG, and ERCC1-XPF factors and a closed-circular plasmid containing a single Pt-GTG-DNA cross-link as a template or in a reconstituted transcription assay (Tx) composed of recombinant TFIIB, TFIIF, TBP, TFIIE factors, the purified RNA Pol II, and the adenovirus major late promoter template (11). The sizes of the incision products or transcripts are indicated. nt, nucleotides.