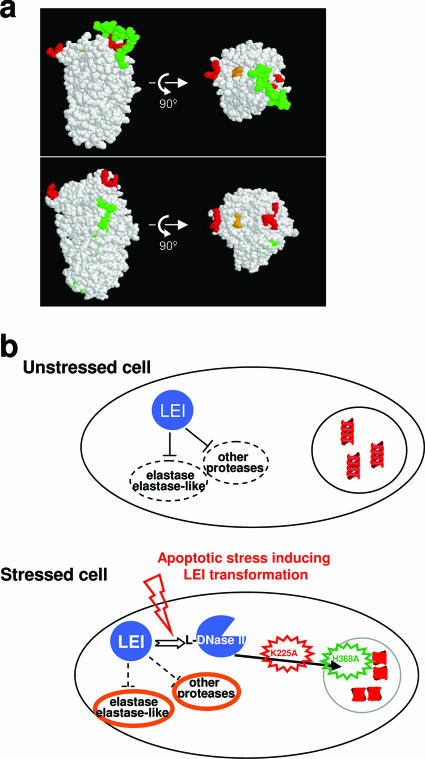
FIG. 5.
Molecular structure of LEI and its implication in cellular fate. (a) The structure of LEI was analyzed with Protein Explorer. Native LEI (upper part) shows the RCL (green) covering the major cluster of lysines of the bipartite NLS (red). The endonuclease active site is orange. This three-dimensional structure was obtained from the pig LEI sequence. The lower part of the panel shows cleaved horse LEI (PDB number 1HLE) with an unmasked NLS (red) after insertion of the RCL into β-sheet A of serpin. (b) In unstressed cells, LEI is localized in the cytoplasm and has an antiprotease activity that preferentially inhibits elastase. When the cell undergoes a stress that causes the transformation of LEI into L-DNase II, the antiprotease activity is released. L-DNase II enters the nucleus, cleaves the DNA, and finally leads to apoptosis. Different mutant forms of LEI have been constructed to understand the LEI/L-DNase II pathway. NLS mutant LEI (K225A, red) is not able to enter the nucleus, and endonuclease active-site mutant LEI (H368A, green) is able to enter the nucleus but is unable to degrade DNA. In both cases, apoptosis is reduced. LEI has to be cleaved into L-DNase II and translocated to the nucleus to induce apoptosis.