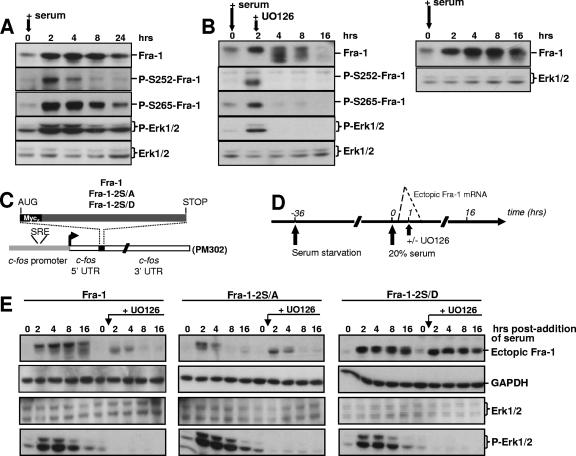
FIG. 6.
Wild-type and mutant Fra-1 stability during a G0/G1-to-S-phase transition. (A) Phosphorylation of Fra-1 in serum-stimulated cells. BALB/c 3T3 fibroblasts were brought in G0 phase by serum deprivation for 36 h. They were then stimulated for growth by the readdition of culture medium containing 20% serum. Immunoblotting experiments were conducted with extracts from cells stimulated for various periods of time with the indicated antibodies. GAPDH (not shown) and Erk1/2 were used as an invariant internal standards. (B) Fate and phosphorylation of Fra-1 in serum-stimulated cells treated with UO126. In the left panel, cells were treated as described above (A), except that UO126 was added 2 h after stimulation with serum. The right panel corresponds to control cells treated in parallel with no UO126 addition and allows the visualization of the faster Fra-1 decay in the presence of UO126. (C) Structure of transient expression vectors. Fra-1, Fra-1-2S/A, and Fra-1-2S/D open reading frames were cloned in the PM302 vector after the removal of its original c-Fos insert (1). They were stably transfected in BALB/c 3T3 fibroblasts. UTR, untranslated region; SRE, serum-responsive element. (D) Design of the synchronization experiment. Deprivation of and stimulation by serum of the various cells stably transfected with the plasmids described in C were performed as described in A. When required, UO126 was added 1 h poststimulation, which gives sufficient time for Fra-1 accumulation. Ectopic mRNA levels peaked by 45 to 60 min and were back to the basal level by 90 to 120 min after serum addition (1). (E) Immunoblotting assays. Immunoblotting experiments were carried out as described above (A) on the cells stably transfected with the various PM302-based vectors. The data presented are representative of at least three independent experiments.