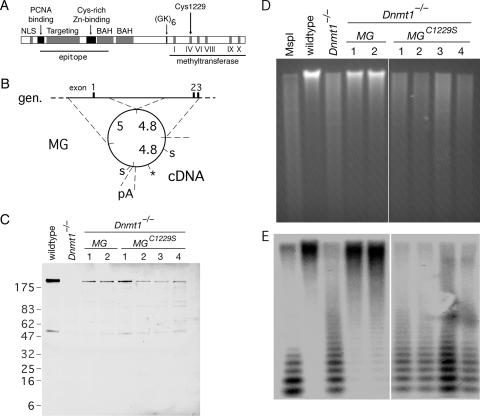
FIG. 1.
A point mutation in DNMT1 abolishes methyltransferase activity. (A) Domains of DNMT1 include the nuclear localization signal (NLS), PCNA binding domain, targeting to replication foci, cysteine-rich Zn2+-binding domain, bromo-adjacent homology domain (BAH), a glycine- and lysine-rich segment (GK), and the methyltransferase domain with diagnostic motifs. A large segment of the amino terminus served as an epitope for the anti-DNMT1 (pATH52) antibody (4). A conservative cysteine→serine mutation at residue 1229 (C1229S) was introduced into a murine Dnmt1 minigene. (B) Schematic of the Dnmt1 minigene (MG) described previously by Tucker et al. (56). The minigene is comprised of two genomic (gen.) fragments that contain a regulatory sequence and the first three exons, cDNA that encodes the remainder of the protein, and a polyadenylation sequence (pA) from the PGK locus. The lengths of these fragments (in kb) are shown. To generate the C1229S mutation (marked by the asterisk), the 4.4-kb fragment between SalI sites (“S”) was subcloned into a cloning vector, subjected to site-directed mutagenesis, and subcloned back into the minigene. (C) The Dnmt1 minigene or the Dnmt1C1229S minigene was electroporated into Dnmt1−/− ES cells to establish stable cell lines in which the minigene construct was the only source of DNMT1. Cell lysates from wild-type cells, Dnmt1−/− cells, two clones of Dnmt1−/− MG, and four clones of Dnmt1−/− MGC1229S were resolved on a 10 to 20% gradient gel and immunoblotted with anti-DNMT1 (pATH52) antibody. Some nonspecific bands are apparent. (D) Ethidium-stained gel of HpaII-digested genomic DNA from the panel of cell lines shown in C. HpaII is sensitive to CpG methylation, but isoschizomer MspI is not. (E) Southern blot of gel in D with a probe to the minor satellite (centromeric) repeats.