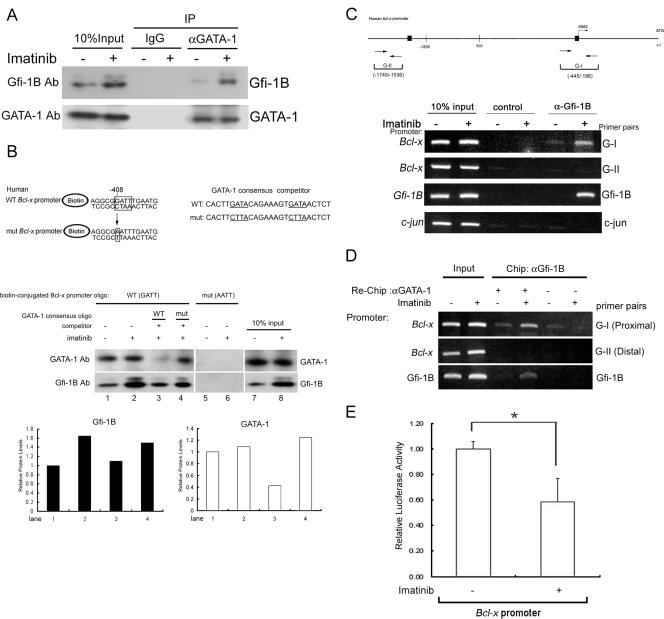
FIG. 6.
Imatinib-induced Gfi-1B formed a complex with GATA-1 on the Bcl-x promoter. (A) K562 cells were treated with or without imatinib (1 μM). After 24 h, whole-cell lysates were prepared and immunoprecipitated with anti-GATA-1 antibody (αGATA-1) or control IgG. The immunoprecipitates (IP) were subjected to immunoblot analysis with anti-Gfi-1B antibody (Ab), followed by reprobing with anti-GATA-1 antibody to confirm that GATA-1 was successfully immunoprecipitated. −, absent; +, present. (B) Pulldown assay using biotin-labeled oligonucleotides containing the Gfi-1B binding sequence (−408 to −405) on the human Bcl-x promoter. Nuclear extracts prepared from untreated or imatinib-treated K562 cells were incubated with biotin-labeled double-stranded oligonucleotides containing the wild-type (WT) or mutated (mut) Gfi-1B binding site. The bound proteins were pulled down with streptavidin-agarose and were analyzed by immunoblotting using antibody against Gfi-1B or GATA-1. Competitor oligonucleotides (oligo) specific for typical and mutated GATA-1 canonical consensus sequences were each included in the reaction mixture as indicated (lanes 3 and 4). Relative amounts of GATA-1 and Gfi-1B protein in lanes 1 to 4 were evaluated by densitometric determination and are shown in the lower panel. (C) In vivo cross-linked protein-DNA complexes isolated from untreated and imatinib-treated K562 cells were subjected to immunoprecipitation with IgG (control) or Gfi-1B antibody (α-Gfi-1B) as indicated. The coimmunoprecipitated DNA was amplified by PCR using the Bcl-x primers G-I (−445 to −196) and G-II (−1745 to −1536), the primer pair for c-jun, or the primer pair specific for the Gfi-1B promoter. Putative Gfi-1 and Gfi-1B binding sites are shown by boxes. The PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. (D) Following ChIP with Gfi-1B antibody, the complexes were subjected to a second immunoprecipitation (re-ChIP) with either anti-GATA-1 antibody (αGATA-1) or control IgG. The coimmunoprecipitated DNA was amplified by PCR using the indicated primers: G-I, G-II, the primer pair specific for the Gfi-1B promoter, or the c-jun primer pair as a control. (E) K562 cells were transfected with the Bcl-x promoter construct, pGL2-3.2, together with pRL-TK. After 24 h, cells were treated with or without 1 μM imatinib for an additional 24 h. Cells were then harvested for the luciferase assay. Values are the averages of three independent determinations. The error bars represent standard deviations. *, P < 0.05.