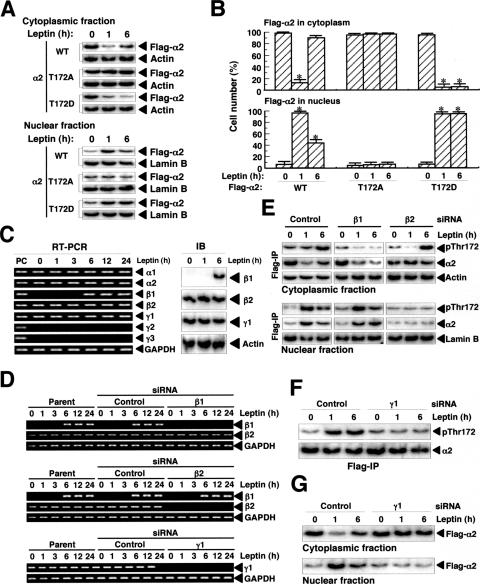
FIG. 4.
Phosphorylation of α2 on Thr172 and the presence of β and γ regulatory subunits are necessary for the leptin-induced changes in the subcellular localization of α2AMPK in C2C12 cells. (A) Cells transiently expressing Flag-α2 (WT, T172D, or T172A) were treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was then subjected to immunoblot analysis with antibodies to Flag, to β-actin, or to lamin B, as indicated. (B) Cells transiently expressing Flag-α2 (WT, T172A, or T172D) were treated with leptin for the indicated times, fixed, and immunostained with anti-Flag. The numbers of cells in which Flag-α2 was detected in the cytoplasm or in the nucleus were counted (total of 500 cells per well). Data are means ± SEM (error bars) from three independent experiments. *, P was <0.05 versus the corresponding value for time zero. (C) Cells were treated with leptin for the indicated times, after which total RNA was isolated and subjected to RT-PCR analysis of AMPK-subunit and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs (left panel); total RNA from mouse skeletal muscle (mixture of soleus and red and white types of gastrocnemius) was used as a positive control (PC). Alternatively, extracts of the leptin-treated cells were subjected to immunoblot (IB) analysis with antibodies to β1, β2, or γ1 subunits of AMPK and to β-actin. (D) Cells transfected with control, β1, β2, or γ1 siRNAs or parental cells were treated with leptin for the indicated times, after which total RNA was isolated and subjected to RT-PCR analysis of β1, β2, γ1, or GAPDH mRNAs. (E) Cells stably expressing Flag-α2 were transfected with control, β1, or β2 siRNAs and then treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. These fractions were subjected to immunoblot analysis with antibodies to β-actin or to lamin B, respectively. The fractions were also subjected to immunoprecipitation (IP) with anti-Flag, and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated α subunits of AMPK and to α2. (F) Cells stably expressing Flag-α2 were transfected with control or γ1 siRNAs and treated with leptin for the indicated times, after which cell extracts were subjected to immunoprecipitation with anti-Flag and the resulting precipitates were subjected to immunoblot analysis with antibodies to Thr172-phosphorylated α subunits of AMPK and to α2. (G) Cells stably expressing Flag-α2 were transfected with control or γ1 siRNAs and treated with leptin for the indicated times, after which cell lysates were separated into cytoplasmic and nuclear fractions. Each fraction was subjected to immunoblot analysis with anti-Flag.