Table 1.
Synthesis and Structures of Lavendamycins
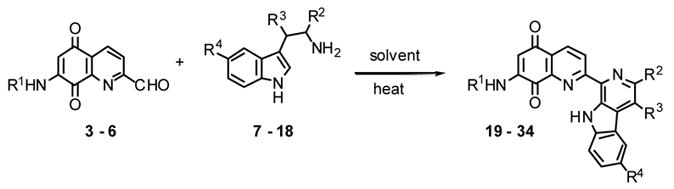
| No. | R1 | R2 | R3 | R4 | % yield | hrs (°C )a |
|---|---|---|---|---|---|---|
| 19 | CH3CO | CO2C2H5 | H | H | 55 | 3 (25-reflux), 19 (reflux) |
| 20 | CH3CO | CO2CH(CH2)5 | H | H | 59 | 3 (25-reflux), 1(reflux) |
| 21 | CH3CO | CO2C5H11-i | CH3 | H | 59 | 3 (<140), 1 (140) |
| 22 | CH3CO | CO2(CH2)2N(CH3)2 | H | H | 20 | 27 (100) |
| 23 | CH3CO | CO2(CH2)2N(CH3)2 | CH3 | H | 36 | 5.5 (100) |
| 24 | CH3CO | CO2C4H9-n | H | OCH3 | 66 | 6 (70-90) |
| 25 | CH3CO | CO2C4H9-n | H | F | 51 | 5 (25–150) |
| 26 | ClCH2CO | CO2CH3 | CH3 | H | 46 | 3 (25–135), 16 (135) |
| 27 | ClCH2CO | CO2C4H9-n | H | H | 20 | 3 (76), 5.75 (76) |
| 28 | ClCH2CO | CO2C8H17-n | H | H | 53 | 6 25–100), 5.5 (100) |
| 29 | n-C3H7CO | CO2CH3 | H | H | 59 | 3 (25–130), 22 (130) |
| 30 | n-C3H7CO | CO2CH3 | CH3 | H | 44 | 3 (25–130), 16 (130) |
| 31 | n-C3H7CO | CO2C5H11-i | H | H | 70 | 4 (25-reflux), 17 (reflux) |
| 32 | n-C3H7CO | CO2C8H17-n | H | H | 80 | 5 (25–85), 16 (85) |
| 33 | i-C3H7CO | CO2CH3 | H | H | 63 | 3 (25–130), 4 (130) |
| 34 | i-C3H7CO | CO2C4H9-n | H | H | 65 | 5(25-125), 3 (125) |
The reaction solvent used for producing 22-25, 27 and 29 was dry anisole and for the remainder dry xylene was used.
