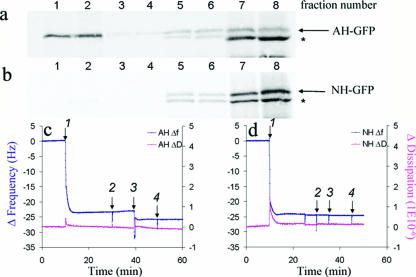
FIG. 1.
NS5A amphipathic alpha-helix-mediated membrane association to model lipid membranes. (a and b) Biochemical membrane flotation analysis of the binding of AH-GFP or NH-GFP proteins to POPC vesicles. POPC vesicles were formed from extrusion through 30-nm track-etched membranes. The effective diameter of the vesicles, as measured by dynamic light scattering, was 65 nm ± 1.2 nm. In vitro-translated AH-GFP (consisting of the NS5A AH with an in-frame C-terminally fused GFP tag) and NH-GFP (same as AH-GFP except that it contains three mutations designed to disrupt the hydrophobic face of the AH [3]) proteins were then mixed with the POPC lipids and subjected to the flotation assay as described previously (3). Note the association of AH-GFP, but not NH-GFP, with the low-density membrane-containing fractions (1 and 2 from the top of the gradient). See text for details. Asterisks indicate a shorter form of the AH-GFP protein. (c) QCM-D analysis of AH peptide binding to quartz crystals coated with POPC model lipid membranes. Bilayers were formed on the SiO2 nanosensors from POPC vesicles prepared as described above. In order to make complete bilayers, we utilized a high NaCl concentration (250 mM) in a PBS buffer. Frequency [Δf(t)] and dissipation [ΔD(t)] changes detected by QCM-D as a function of time were recorded. At 10 min, 0.05 mg/ml of vesicle solution was injected (arrow 1) followed by thorough washing with PBS buffer (arrow 2). After the frequency as well as the dissipation were stabilized at 40 min, 0.1 mg/ml of AH peptide was injected (arrow 3) to investigate the ability of the AH peptide to bind to the model POPC membrane. To ensure that the AH peptide was indeed bound to the membrane, the latter was washed again with PBS buffer (arrow 4) at 50 min. Note the decrease in resonant frequency change associated with the addition of AH peptide, indicating binding. (d) QCM-D analysis of NH peptide binding to POPC model lipid bilayers. Same as above, except that the AH peptide was replaced with NH peptide. The latter differs from AH in that NH contains three mutations designed to disrupt the hydrophobic face of AH (3). Note that the NH peptide does not bind to model POPC membranes, as indicated by lack of both frequency and dissipation changes. Also note that the vertical spikes are due to the injection of materials into the QCM-D sensor chamber and do not affect the changes in frequency and dissipation recorded before and after injections.