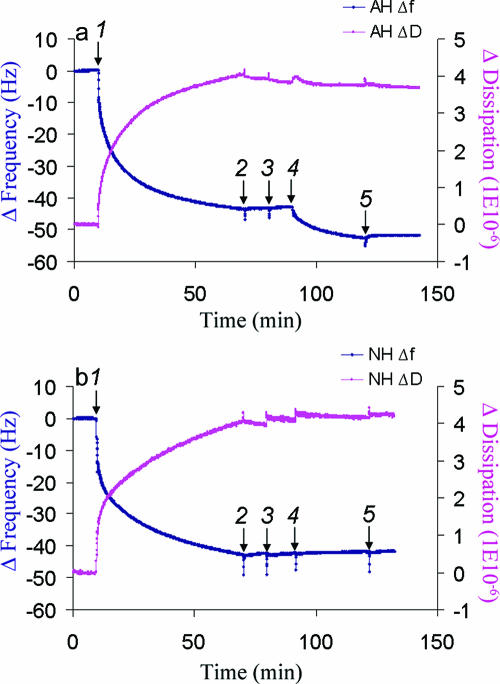
FIG. 2.
QCM-D analysis of peptide binding to Huh7-derived membranes. (a) Binding of AH peptide to Huh7-derived membranes formed on SiO2 quartz crystals and detected by Δf(t) and ΔD(t) changes. At 10 min, Huh7 membrane solution (0.25 mg/ml) was injected (arrow 1). After buffer washes at 70 min and again at 80 min (arrows 2 and 3), 0.05 mg/ml of AH peptide was injected at 90 min (arrow 4). In order to confirm the binding of the peptide, an additional wash with the same buffer was performed (arrow 5). The data show that isolated membranes from the cultured Huh7 cells saturated at a higher mass than the model POPC membrane, presumably due to the presence of proteinaceous components. They also show by the dissipation value that those proteinaceous components are associated with higher viscoelastic energy dissipation, as expected. (b) No binding of NH peptide to Huh7-derived membranes formed on a SiO2 surface and detected by Δf(t) and ΔD(t) changes. NH peptide binding was attempted using the same method as in panel a. Membranes derived from cultured Huh7 cells were injected after 10 min (arrow 1). After waiting for saturation binding of membranes to the crystal, the latter was washed with buffer at 70 min and at 80 min (arrows 2 and 3) followed by the application of 0.05 mg/ml of NH peptide at 90 min (arrow 4) and an additional wash with the same buffer (arrow 5). Note that there are no changes in either the frequency or dissipation, which suggests that there is no binding to Huh7-derived membranes.