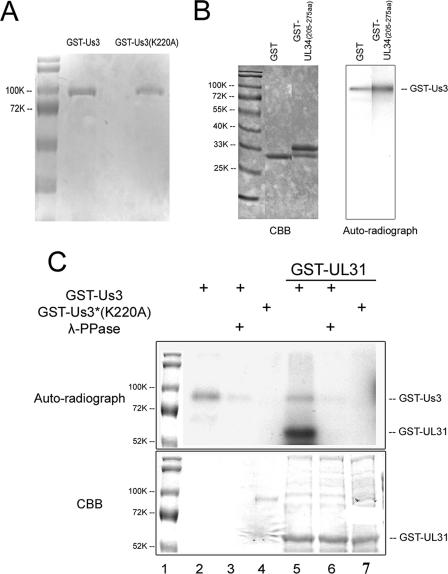
FIG. 3.
Analysis of in vitro kinase activities of purified GST-pUS3 and the mutant GST-pUS3(K220A). (A) Denaturing polyacrylamide gel containing purified GST-pUS3 and GST-pUS3(K220A) and stained with Coomassie brilliant blue. (B) In vitro kinase reaction. The fusion proteins indicated at the top of the left panel were mixed with 100 ng GST-US3. After 30 min at 30°C in the presence of [γ-32P]ATP, the reaction mixtures were resolved on a denaturing polyacrylamide gel and visualized by Coomassie (CBB) staining (left panel) and autoradiography (right panel). The position of the approximately 90,000 apparent Mr GST-US3 fusion protein is shown in the right panel. GST-UL34 (amino acids 205 to 275) is a negative control inasmuch as it does not contain the phosphorylation consensus site in pUL34. (C) In vitro kinase activities of GST-pUS3 and GST-pUS3(K220A). Purified GST-pUS3 (0.1 μg) or 0.5 μg GST-pUS3(K220A) was incubated with [γ-32P]ATP in the presence or absence of partially purified GST-pUL31 as a potential substrate, followed by subsequent incubation in the presence or absence of λ-Ppase. The reaction components were denatured, electrophoretically separated, and stained with Coomassie brilliant blue (CBB, bottom panel) or dried and autoradiographed (upper panel). For comparative purposes, the molecular weight standards from the bottom panel were copied and aligned with the top panel, using Adobe Photoshop. Sizes of the standards are indicated to the left of the figure in thousands [K]).