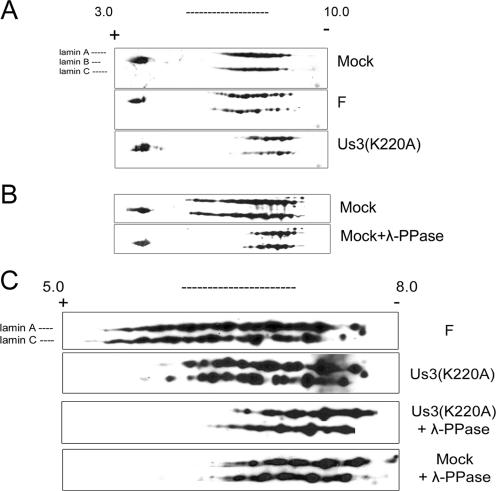
FIG. 7.
Two-dimensional gel electrophoresis and immunoblots of lamin A/C isoforms from mock-infected and HSV-infected cells. Hep2 cells were mock infected or infected with wild-type HSV-1(F) (panels labeled F) or mutant Us3(K220A). Proteins were extracted in buffer containing 9 M urea to solubilize lamins and separated by isoelectric focusing in the first dimension using nonlinear pH gradients from pH 3.0 to 10.0 (A and B) or linear pH gradients 5.0 to 8.0 (C) and by size on denaturing polyacrylamide gels in the second dimension. The proteins were then transferred to nitrocellulose and probed with polyclonal chicken antibody directed against lamin A/C or a rabbit antibody against lamin B1 and lamin B2. Bound immunoglobulin was detected by appropriate horseradish peroxidase-conjugated secondary antibodies. Chemiluminescence generated by the addition of appropriate substrates was recorded on X-ray film. In some experiments (λ-Ppase), proteins were reacted with λ-Ppase before electrophoretic separation. For comparative purposes, the three blots in panels A and B were aligned by position of lamin B-specific spots and by distance from the cationic and anionic poles. The positions of the terminal poles on the pH strip of the four immunoblots shown in panel C are also aligned. For orientation, the positions of the lamin A-, B-, and C-specific spots are indicated to the left of the top immunoblot shown in panels A and C.