FIG. 2.
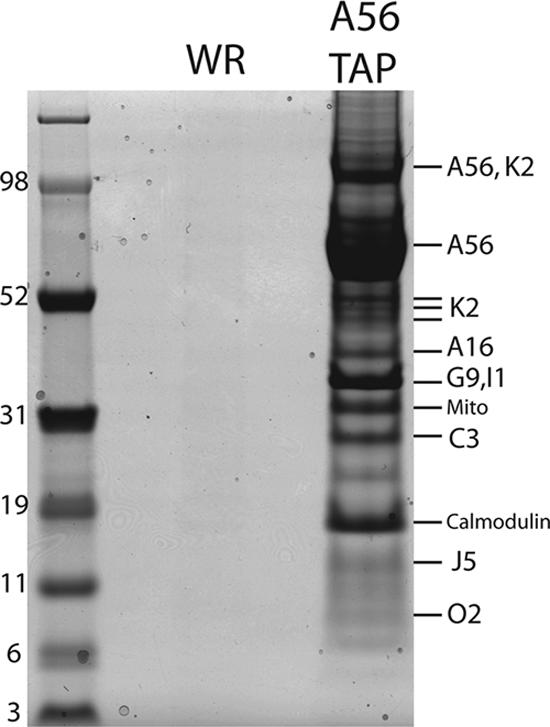
Identification by mass spectrometry of proteins that copurify with A56TAP. HeLa cells were infected with vA56TAP or VACV WR and lysed with Triton X-100, and the postnuclear supernatants were purified successively on streptavidin and calmodulin affinity columns. The bound proteins were eluted, concentrated, resolved by SDS-PAGE, and detected by staining with Coomassie blue. The protein bands were cut from the gel, digested with trypsin, and analyzed by mass spectrometry. Protein identities are indicated to the right of the stained bands. A56 and K2 were resolved as individual polypeptides and in an undissociated heterodimer. Peptides corresponding to two proteins (G9 and I1) were obtained from the same band. The band marked Mito contained peptides corresponding to the mitochondrial protein ATP synthase (gi: 4885079) and solute carrier family 25 member proteins (gi: 32189336). Marker proteins with masses in kilodaltons are indicated at the left.
