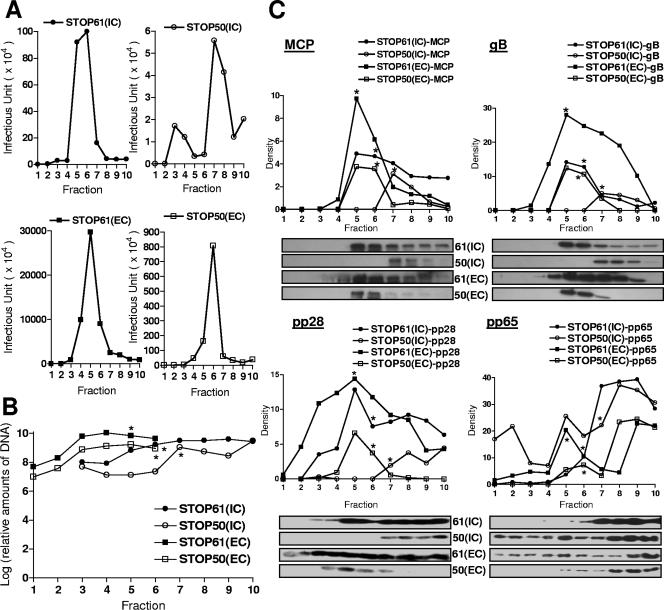
FIG. 6.
Gradient separation and analysis of extracellular and intracellular viruses from pp28STOP61- and pp28STOP50-infected cells. (A) Intracellular (IC) and extracellular (EC) particles were prepared as described in Materials and Methods and fractionated on 20 to 70% sorbitol gradients as described previously (6). The infectivity titers of gradient fractions (1 ml) collected from the bottom of density gradients were determined as described in Materials and Methods. (B) Real-time PCR of viral genome copy numbers in fractions from gradients. Fifty microliters of each fraction was extracted using column technology (QIAGEN blood PCR kit; QIAGEN, Valencia, CA) and assayed by real-time PCR as described in Materials and Methods. Results are expressed as genome copy number per fraction. (C) Relative expression of MCP, gB, pp28, and pp65 in each fraction as detected by Western blotting with specific MAbs. Fifty microliters of each fraction was loaded on 12% polyacrylamide gels and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting as described in Materials and Methods. Following development with enhanced chemiluminescence, densitometry was carried out and the relative signal for each lane was determined. Note that densitometry was done only with fluorograms acquired during the same experiment and developed for the same time interval. The fluorograms shown for pp28 and pp65 were processed in Adobe Photoshop to remove nonspecific background signals assumed to be secondary to nonspecific binding of primary antibodies; however, densitometry was performed prior to image processing to remove background. The pp65- and pp28-specific bands were not processed. *, gradient fraction with peak infectivity.