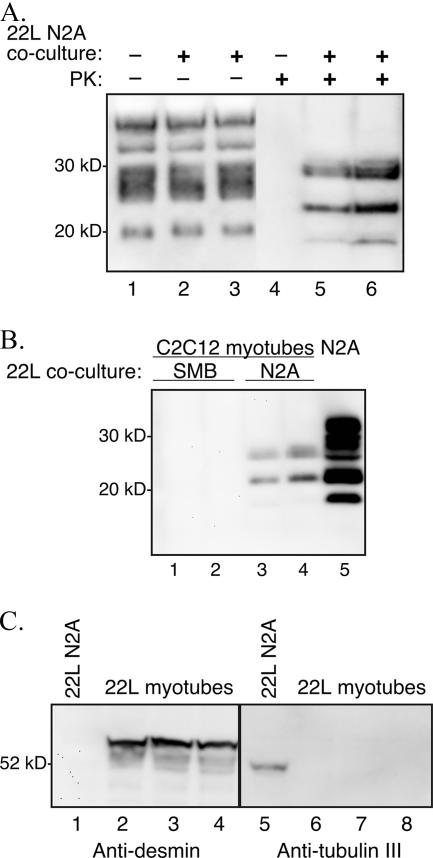
FIG. 2.
Coculture of C2C12 myoblasts and 22L scrapie-infected N2A cells. C2C12 myoblasts containing a plasmid expressing Zeocin resistance and 22L scrapie-infected N2A cells were cocultured at a ratio of 1:2 for 1 week prior to selection in Zeocin-containing culture medium. The surviving C2C12/Zeor myotubes were assayed for PrPSc by Western blotting after serial passage in vitro. (A) Cell lysates from C2C12 D5 myotubes at passage 10 in Zeocin-containing selection medium following coculture with either uninfected N2A cells (lanes 1 and 4) or 22L scrapie-infected N2A cells (lanes 2, 3, 5, and 6). The cellular lysates were either undigested (−) or digested (+) with 0.6 U/ml of PK prior to analysis. (B) Western blot of PK-digested lysates from C2C12 D5 myotubes at passage 6 in Zeocin-containing selection medium following coculture with either 22L scrapie-infected SMB cells (lanes 1 and 2) or 22L scrapie-infected N2A cells (lanes 3 and 4). Control 22L N2A cells (lane 5) were in the absence of C2C12 cell coculture and Zeocin selection. (C) Western blotting for desmin and tubulin III was performed on cell lysates from C2C12 D6 myotubes following coculture of 22L scrapie-infected N2A cells and C2C12 myoblasts at passage 4 in Zeocin selection medium (lanes 2 to 4 and 6 to 8). Eighty-six micrograms of 22L scrapie-infected N2A cells (lanes 1 and 5) and 22L scrapie-infected C2C12 D6 myotubes was analyzed using anti-PrP 6H4 monoclonal antibody. Molecular mass markers are shown on the left.