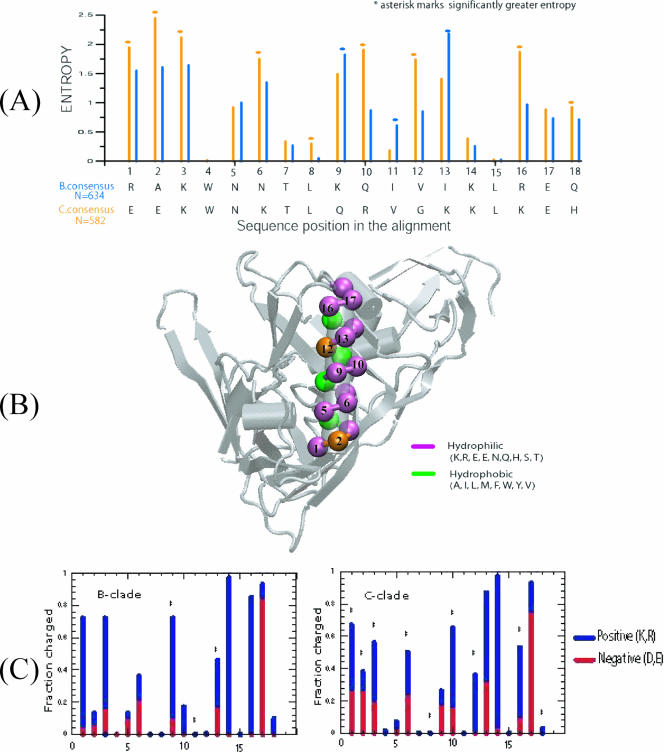
FIG. 1.
Sequence and structural characteristics of α2-helix from the C3 region of gp120 from clades B and C. (A) Sequence entropy profiles of α2-helix for clades B (blue) and C (orange). Sequence entropy at each position in the alignment is evaluated (582 C-clade and 634 B-clade sequences), and the consensus sequences are also shown for completeness. The asterisk marks significantly greater entropy. Positions 1, 2, 3, 6, 8, 10, 12, 16, and 18 exhibit statistically significantly higher sequence entropy scores in clade C; only positions 9 and 13 are found to have greater variability in clade B. Positions 4, 7, 8, 14, and 15 in both clades and position 11 within individual clades are conserved and are hydrophobic in nature. Substitutions at these positions also conform to maintain the hydrophobicity, as seen in positions 8 and 11, which show slight variability in clades C and B, respectively. (B) Mapping of residue positions from panel A onto the X-ray structure of HXB2 (10). The hydrophobic and hydrophilic residues are marked by green and violet, respectively. The dark amber colors in position 2 and 12 mark the differences in residue types between the clades. In clade C, those positions are hydrophilic, whereas in clade B, position 12 is hydrophobic and position 2 can either be hydrophobic or hydrophilic. In this X-ray structure, the V4 loop was not resolved (10). (C) Fraction of charged residues (blue, positive; red, negative) at each position of the α2-helix of clades B and C as identified in panel A.