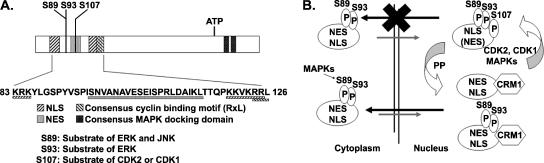
FIG. 5.
Mechanisms governing HPV-11 E1 nucleocytoplasmic localization. (A) Interdigitated regulatory elements in the LRR near the amino terminus of the HPV-11 E1 protein that control its subcellular localization, as identified in the present study and in our previous report (27, 48). (B) Model for mechanisms governing E1 nucleocytoplasmic shuttling. The E1 protein is largely cytoplasmic until signal transductions activate MAPKs, in particular ERK and JNK, which in turn activate the E1 NLS by phosphorylating S89 and S93. Once imported into the nucleus, the protein is efficiently exported back to the cytoplasm by the dominant CRM-1-dependent NES unless its NES is inactivated upon phosphorylation of S107 by cdk2 or cdk1, kinases that are available during S and G2 phase in the presence of the appropriate cyclins. When the cell exits the G2 phase or the cell cycle all together, E1 dephosphorylated on S107 by protein phosphatases is exported by the potent NES out of the nucleus. Thus, E1 nuclear retention is coupled to viral DNA replication and the cell cycle so that it will not unwind the viral DNA when cells cannot support viral DNA replication for lack of host DNA replication machinery or substrates.