FIG. 6.
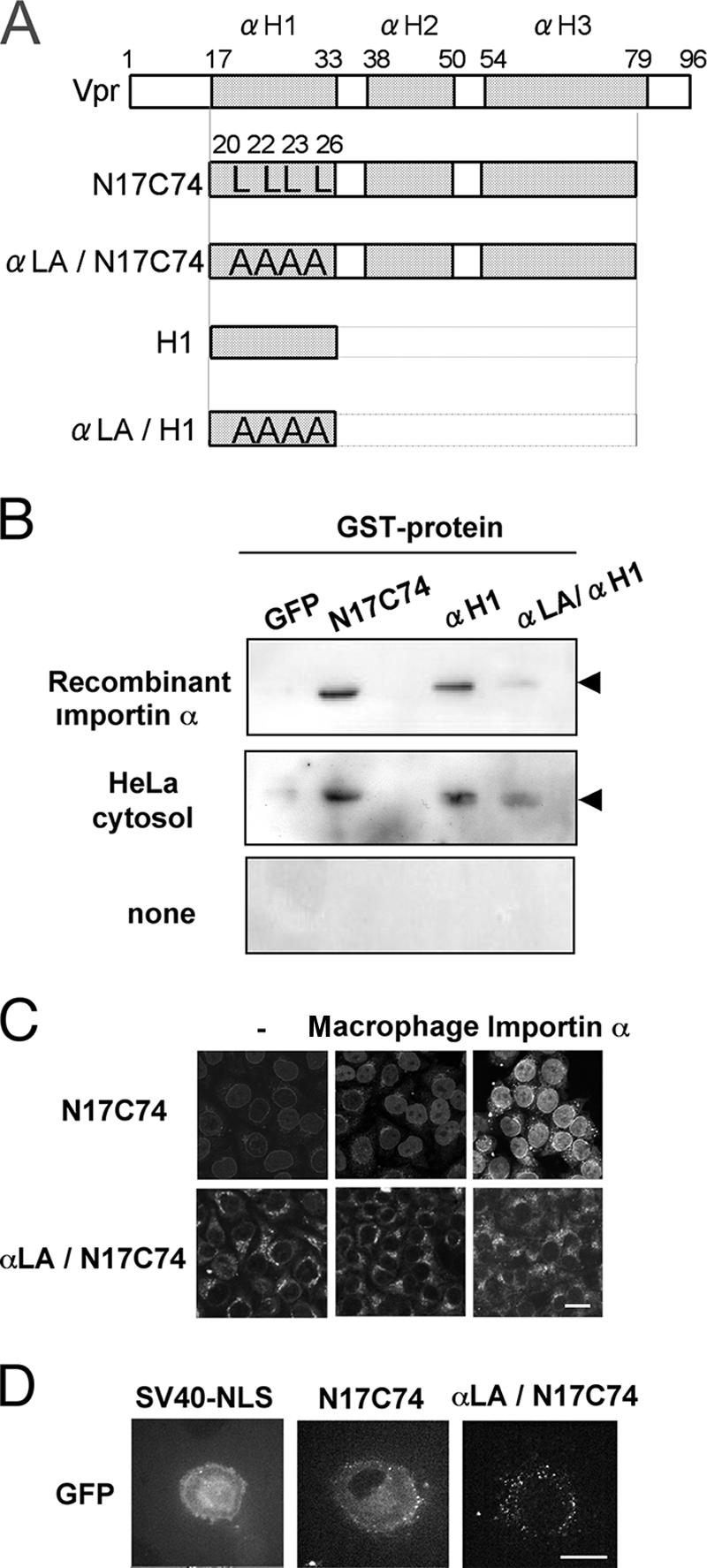
Vpr mutants that cannot bind importin α are not imported into the nucleus. (A) Construction of plasmids encoding GST- and GFP-tagged mutant forms of Vpr. The three α-helical domains (αH1, αH2, and αH3) are represented by shaded boxes. The Leu residues at positions 20, 22, 23, and 26 in the αH1 domain were replaced by Ala. (B) Glutathione-Sepharose beads were coupled with the recombinant proteins, namely, GST-tagged GFP, GST-N17C74-GFP, GST-αH1-GFP, and GST-αLA/αH1-GFP, and incubated with 100 pmol recombinant importin α (top), 100 μg cytosol of HeLa cells (middle), or nothing as a control (bottom). The proteins recovered from the beads were subjected to Western blotting with a MAb against importin α1. The position of importin α is indicated. (C) Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 or αLA/N17C74 in the presence of 100 μg cytoplasmic extract prepared from primary macrophages or 1 μM recombinant importin α. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm. (D) GST- and GFP-tagged N17C74, αLA/N17C74 or SV40 NLS was injected into the cytoplasm of differentiated primary macrophages grown on a glass-bottomed dish. After 15 min, the transport reactions were captured by confocal laser scanning microscopy. Bar = 20 μm.
