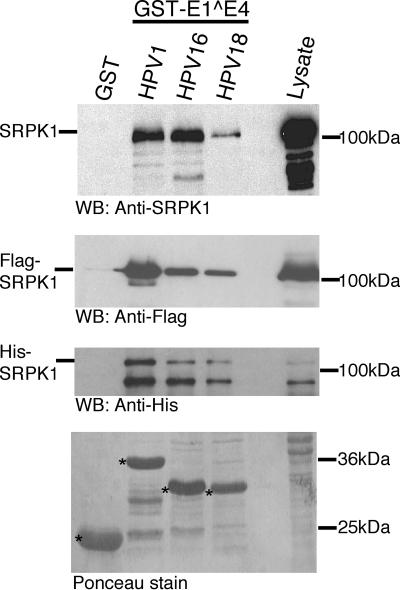
FIG. 3.
The E1^E4 interaction with SRPK1 is conserved between different HPV types. SRPK1 binding to E1^E4 proteins of various HPV types was investigated by using GST-E1^E4 fusion proteins of HPV1, HPV16, and HPV18 to coprecipitate endogenous SRPK1 derived from SV-JD lysates (upper panel), Flag-SRPK1 expressed in 293T cells (middle upper panel), and His-SRPK1 expressed in bacteria (lower middle panel). A Ponceau-stained nitrocellulose membrane used for the SRPK1 coprecipitation demonstrates that the relative amounts of GST and GST fusion proteins (indicated by asterisks) used in these experiments is approximately equal (lower panel). In all cases, SRPK1 forms complexes specifically with all of the different GST-E1^E4 proteins but not GST alone. In the lower middle panel, the upper band migrating with an apparent molecular mass of over 120 kDa represents the full-length His-SRPK1, while the smaller band is likely to be a breakdown product of His-SRPK1. WB, Western blot.