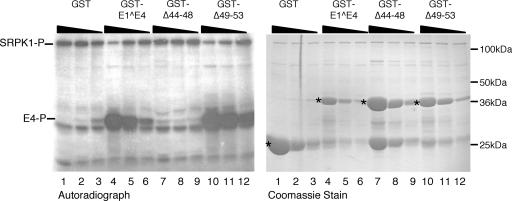
FIG. 7.
In vitro phosphorylation of HPV1 E1^E4 is dependent on SRPK1 binding. In vitro kinase reactions containing His-SRPK1 and GST-HPV1 E1^E4 fusion proteins that bind SRPK1 (E1^E4 and Δ49-53) or are defective for binding (Δ44-48) titrated at 25, 5, and 1 μg. The relative amounts of His-SRPK1 and the different GST proteins (indicated by asterisks) present in the kinase reactions are shown in the Coomassie-stained gel. A major phosphospecies (E4-P) is present in in vitro kinase reaction mixtures containing the wild-type E1^E4 protein (lanes 4 to 6) and the Δ49-53 deletion mutant (lanes 10 to 12) but is not detected in reactions containing the Δ44-48 deletion mutant (lanes 7 to 9) or the GST control protein (lanes 1 to 3). In the titrations of E1^E4 substrates that interact with SRPK1, we observed a dose-dependent reduction in autophosphorylation of the full-length His-SRPK1 polypeptide (SRPK1-P).