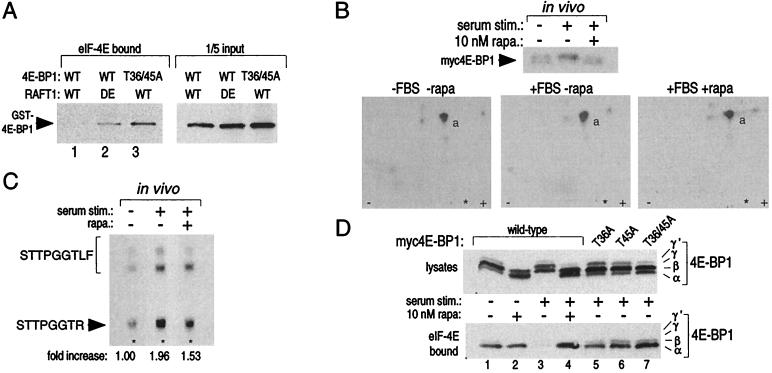
Figure 5.
Thr-36 and Thr-45 are phosphorylated in vivo, and mutations of these residues to alanine create a 4E-BP1 variant that binds constitutively to eIF-4E in vitro and in vivo. (A) 4E-BP1 phosphorylated in vitro by wild-type (WT) RAFT1, but not by kinase-dead (DE) RAFT, cannot bind to eIF-4E (Left, lanes 1 and 2). Even after an incubation with wild-type RAFT1 in a kinase assay, the T36/45A mutant still binds to eIF-4E (Left, lane 3). Equal amounts of GST-4E-BP1 were offered for binding in each condition (Right). Kinase assays of 30-min duration were performed as in Fig. 1A except that only 100 ng of fusion protein was used. The reaction products were then offered for binding to recombinant eIF-4E prebound to 7-methyl-GTP Sepharose. Bound 4E-BP1 was detected by immunoblotting with an anti-GST antibody. (B) Tryptic peptide maps (Lower) of 4E-BP1 isolated form cells labeled in vivo with 32P (Upper) and treated with the indicated conditions. Peptide “a” is eliminated in maps prepared with a T36/45A mutant (data not shown). (C) Serum stimulation increases phosphate content at Thr-45 2-fold, and rapamycin blocks 50% of the increase. Peptide “a” was purified from the TLC plates of B and analyzed as described in Materials and Methods. The spots were quantitated with densitometry. (D) Alanine mutations at Thr-36 and Thr-45 prevent serum-induced release of 4E-BP1 from eIF-4E and mimic the effects of rapamycin. HEK293 cells were transfected with plasmids encoding wild-type or the indicated mutant 4E-BP1s, treated with rapamycin and serum, and the migration pattern of 4E-BP1 (Upper) or the amount of 4E-BP1 bound to eIF-4E (Lower) was determined by immunoblotting.