Abstract
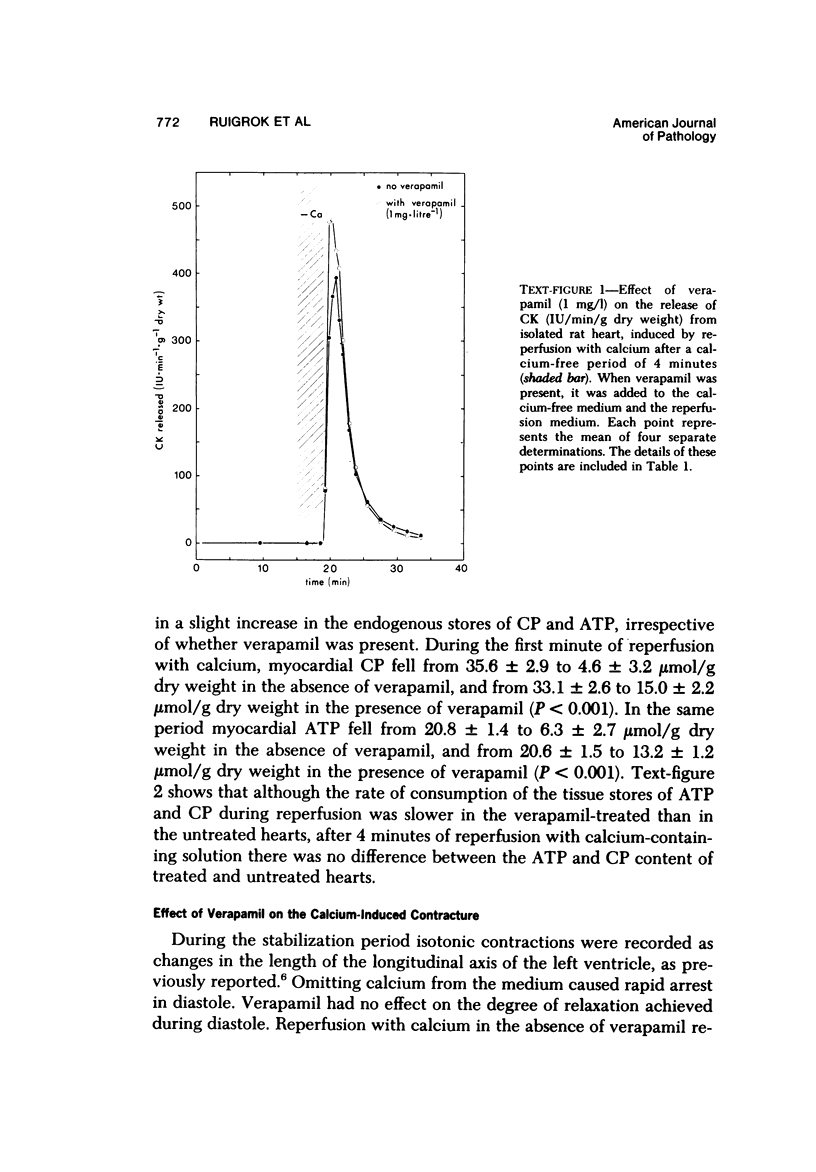
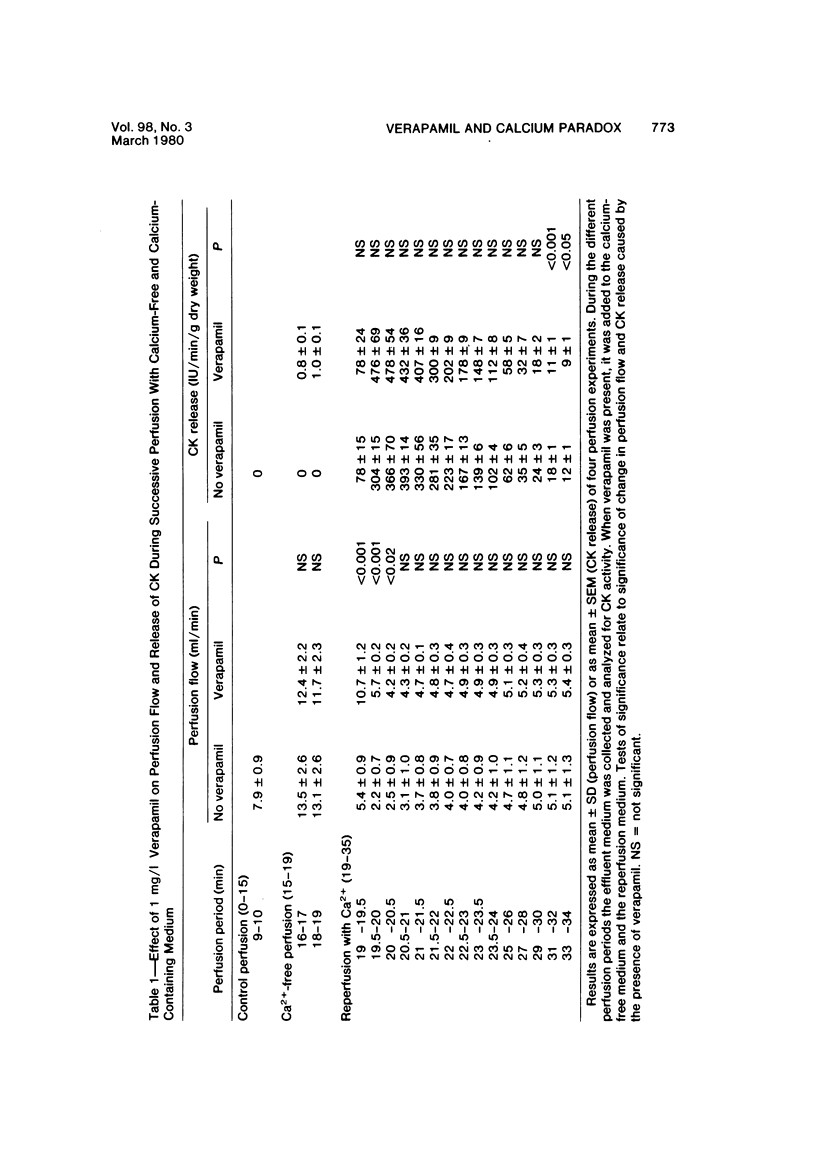
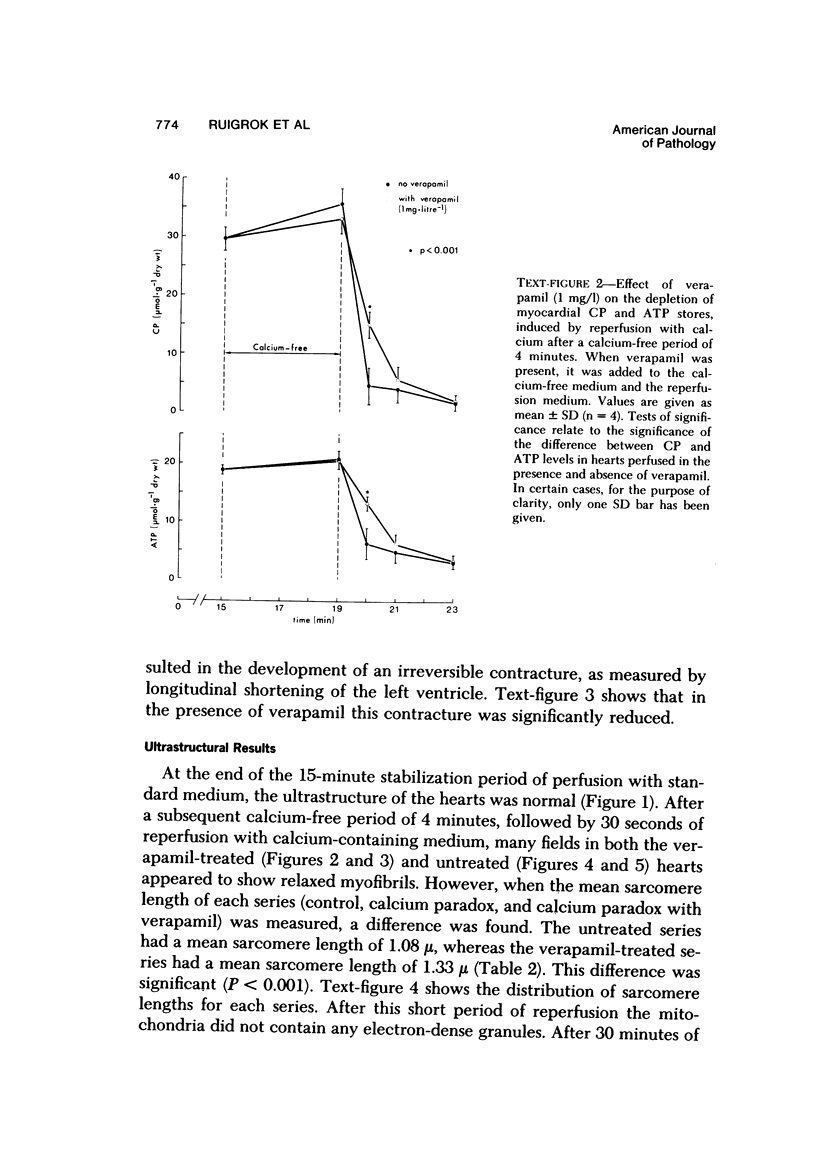
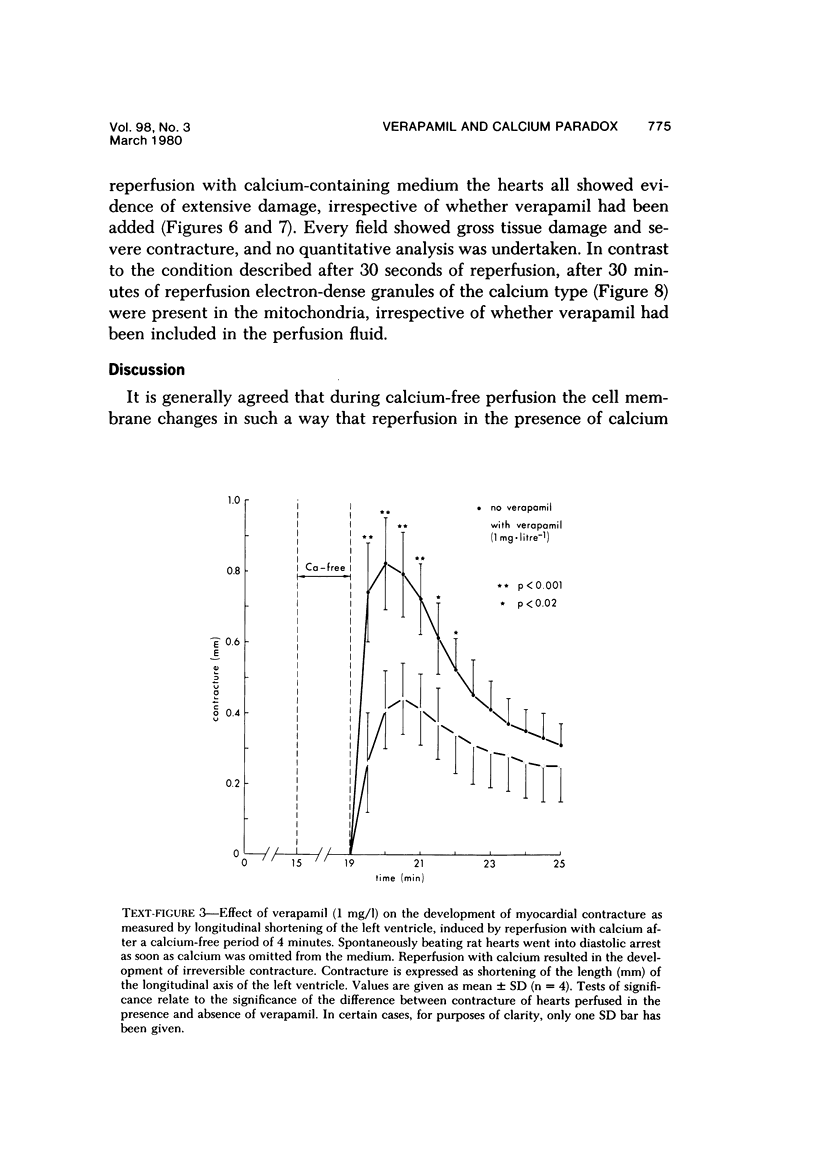
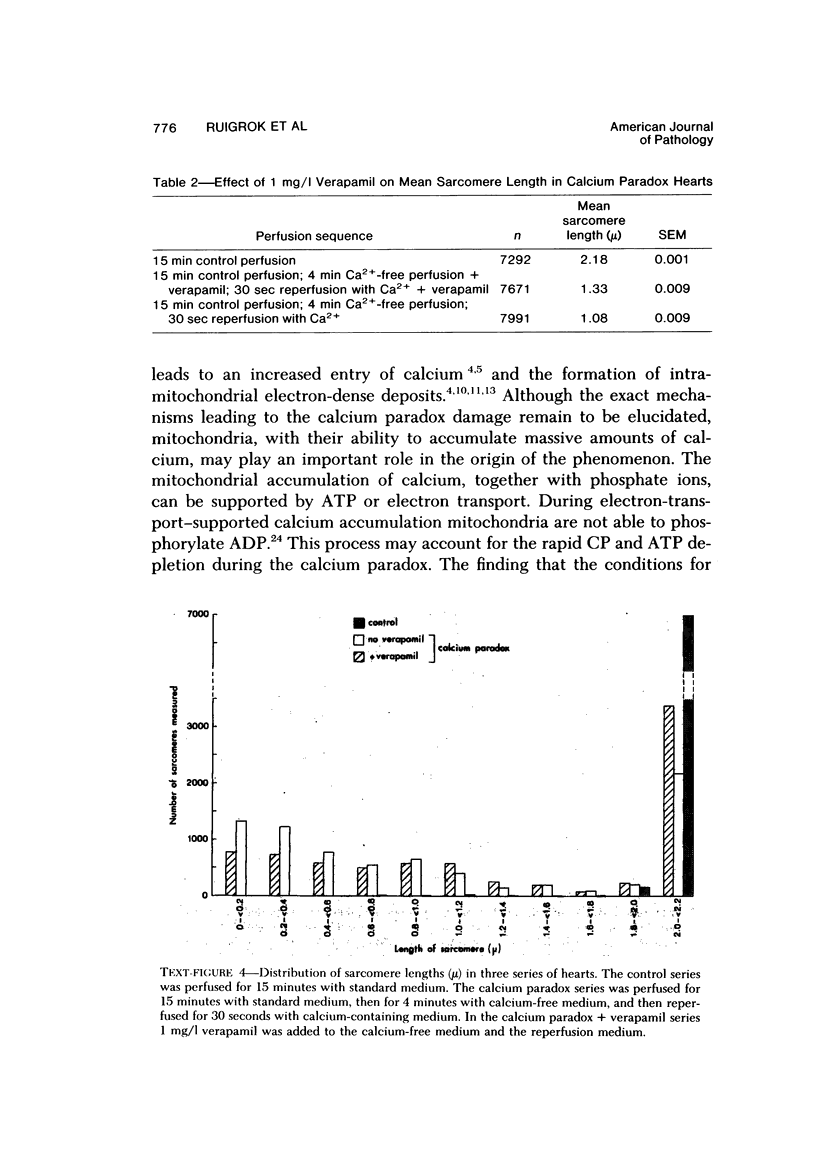
Reperfusion of isolated rat hearts with calcium-containing medium after a short period of calcium-free perfusion results in irreversible cell damage (calcium paradox). Experiments were undertaken to determine whether the slow-channel calcium-antagonist drug verapamil protects calcium-deprived rat heart muscle against the consequences of readmitting calcium. Cell damage was quantitated in terms of creatine kinase (CK) release, depletion of endogenous creatine phosphate (CP) and adenosine triphosphate (ATP) stores, development of contracture as measured by longitudinal shortening of the left ventricle, and ultrastructural damage. Verapamil (1 mg/l) did not reduce the initial rate of CK release during reperfusion with calcium but reduced the initial rate at which myocardial CP and ATP stores were depleted and decreased the shortening of the longitudinal axis of the left ventricle. After 30 seconds of reperfusion the mean sarcomere length was significantly greater in the verapamil-treated hearts. These results can be interpreted to mean that inhibition of calcium inflex via the slow channels does not protect heart muscle against the deleterious effects of readmitting calcium after a period of calcium-free perfusion.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIERLEY G. P., MURER E., BACHMANN E. STUDIES ON ION TRANSPORT. III. THE ACCUMULATION OF CALCIUM AND INORGANIC PHOSPHATE BY HEART MITOCHONDRIA. Arch Biochem Biophys. 1964 Apr;105:89–102. doi: 10.1016/0003-9861(64)90239-5. [DOI] [PubMed] [Google Scholar]
- Bionk A. B., Ruigrok T. J., Maas A. H., Zimmerman A. N. Changes in high-energy phosphate compounds of isolated rat hearts during Ca2+-free perfusion and reperfusion with Ca2+. J Mol Cell Cardiol. 1976 Dec;8(12):973–979. doi: 10.1016/0022-2828(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Bulkley B. H., Nunnally R. L., Hollis D. P. "Calcium paradox" and the effect of varied temperature on its development: a phosphorus nuclear magnetic resonance and morphologic study. Lab Invest. 1978 Aug;39(2):133–140. [PubMed] [Google Scholar]
- Crevey B. J., Langer G. A., Frank J. S. Role of Ca2+ in maintenance of rabbit myocardial cell membrane structural and functional integrity. J Mol Cell Cardiol. 1978 Dec;10(12):1081–1100. doi: 10.1016/0022-2828(78)90354-1. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Tomlinson C. W., Singh J. N., Lee S. L., McNamara D. B., Harrow J. A., Yates J. C. Role of sarcolemmal changes in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab. 1976;9:377–394. [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Worstell J., Kaltenbach J. P. Oxygen-induced enzyme release after irreversible myocardial injury. Effects of cyanide in perfused rat hearts. Am J Pathol. 1976 Aug;84(2):327–350. [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J., Garlick P. B., Humphrey S. M. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol. 1977 Jun;39(7):986–993. doi: 10.1016/s0002-9149(77)80212-9. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Boink A. B., Ruigrok T. J. The calcium paradox: metabolic, electrophysiological, contractile and ultrastructural characteristics in four species. Eur J Cardiol. 1978 Jun;7(4):241–256. [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Schuchleib R., Davis J., Weiss E. S., Sobel B. E. Myocardial contracture and accumulation of mitochondrial calcium in ischemic rabbit heart. Am J Physiol. 1977 Dec;233(6):H677–H684. doi: 10.1152/ajpheart.1977.233.6.H677. [DOI] [PubMed] [Google Scholar]
- Holland C. E., Jr, Olson R. E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975 Dec;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jynge P., Hearse D. J., Braimbridge M. V. Myocardial protection during ischemic cardiac arrest. A possible hazard with calcium-free cardioplegic infusates. J Thorac Cardiovasc Surg. 1977 Jun;73(6):848–855. [PubMed] [Google Scholar]
- Jynge P., Hearse D. J., de Leiris J., Feuvray D., Braimbridge M. V. Protection of the ischemic myocardium. Ultrastructural, enzymatic, and functional assessment of the efficacy of various cardioplegic infusates. J Thorac Cardiovasc Surg. 1978 Jul;76(1):2–15. [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Tada M. The "stone heart" and other challenges to the biochemist. Am J Cardiol. 1977 Jun;39(7):1073–1077. doi: 10.1016/s0002-9149(77)80224-5. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Tada M. The "stone heart": a challenge to the biochemist. Am J Cardiol. 1972 Apr;29(4):578–580. doi: 10.1016/0002-9149(72)90455-9. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Locke F. S., Rosenheim O. Contributions to the physiology of the isolated heart: The consumption of dextrose by mammalian cardiac muscle. J Physiol. 1907 Dec 31;36(4-5):205–220. doi: 10.1113/jphysiol.1907.sp001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIJLER F. L., vd BOGAARD F., vd TWEELH D., DURRER Postextrasystolic potentiation in the isolated rat heart. Am J Physiol. 1962 Apr;202:631–635. doi: 10.1152/ajplegacy.1962.202.4.631. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Grau A., Slade A. A protective effect of verapamil on hypoxic heart muscle. Cardiovasc Res. 1976 Nov;10(6):650–662. doi: 10.1093/cvr/10.6.650. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Krikler D. Verapamil and the myocardium. Postgrad Med J. 1974 Jul;50(585):441–446. doi: 10.1136/pgmj.50.585.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Seabra-Gomes R. Effect of methylprednisolone sodium succinate on hypoxic heart muscle. Cardiovasc Res. 1976 May;10(3):349–358. doi: 10.1093/cvr/10.3.349. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Yepez C., Grau A., Slade A. Protective effect of methylprednisolone sodium succinate on the ultrastructure and resting tension of hypoxic heart muscle. Cardiovasc Res. 1978 Feb;12(2):91–98. doi: 10.1093/cvr/12.2.91. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J Physiol. 1883 Jan;4(1):29–42.3. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Spies F., Blok F. J., Maas A. H., Zimmerman A. N. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978 Nov;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Ruigrok T. J., Burgersdijk F. J., Zimmerman A. N. The calcium paradox: a reaffirmation. Eur J Cardiol. 1975 Jun;3(1):59–63. [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Tyers G. F. Metabolic arrest of the ischemic heart. Ann Thorac Surg. 1975 Jul;20(1):91–94. doi: 10.1016/s0003-4975(10)63858-1. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca2+-free medium. J Mol Cell Cardiol. 1975 Feb;7(2):91–103. doi: 10.1016/0022-2828(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]